Implantable acousto-optic window for monitoring ultrasound-mediated neuromodulation in vivo
- PMID: 35874142
- PMCID: PMC9298854
- DOI: 10.1117/1.NPh.9.3.032203
Implantable acousto-optic window for monitoring ultrasound-mediated neuromodulation in vivo
Abstract
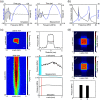
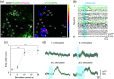
Significance: Ultrasound has recently received considerable attention in neuroscience because it provides noninvasive control of deep brain activity. Although the feasibility of ultrasound stimulation has been reported in preclinical and clinical settings, its mechanistic understanding remains limited. While optical microscopy has become the "gold standard" tool for investigating population-level neural functions in vivo, its application for ultrasound neuromodulation has been technically challenging, as most conventional ultrasonic transducers are not designed to be compatible with optical microscopy. Aim: We aimed to develop a transparent acoustic transducer based on a glass coverslip called the acousto-optic window (AOW), which simultaneously provides ultrasound neuromodulation and microscopic monitoring of neural responses in vivo. Approach: The AOW was fabricated by the serial deposition of transparent acoustic stacks on a circular glass coverslip, comprising a piezoelectric material, polyvinylidene fluoride-trifluoroethylene, and indium-tin-oxide electrodes. The fabricated AOW was implanted into a transgenic neural-activity reporter mouse after open craniotomy. Two-photon microscopy was used to observe neuronal activity in response to ultrasonic stimulation through the AOW. Results: The AOW allowed microscopic imaging of calcium activity in cortical neurons in response to ultrasound stimulation. The optical transparency was over the visible and near-infrared spectra, and the ultrasonic pressure was 0.035 MPa at 10 MHz corresponding to . In anesthetized Gad2-GCaMP6-tdTomato mice, we observed robust ultrasound-evoked activation of inhibitory cortical neurons at depths up to . Conclusions: The AOW is an implantable ultrasonic transducer that is broadly compatible with optical imaging modalities. The AOW will facilitate our understanding of ultrasound neuromodulation in vivo.
Keywords: in vivo two-photon; neuromodulation; ultrasound.
© 2022 The Authors.
Figures


References
-
- Lee J., et al. , “Transcranial magnetic stimulation parameters as neurophysiological biomarkers in Alzheimer’s disease,” Ann. Clin. Neurophysiol. 23, 7–16 (2021). 10.14253/acn.2021.23.1.7 - DOI
-
- Gardner J., “A history of deep brain stimulation: technological innovation and the role of clinical assessment tools,” Soc. Stud. Sci. 43, 707–728 (2013). 10.1177/0306312713483678 - DOI
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

