Synthesis, Mesomorphism, Photophysics, and Device Properties of Liquid-Crystalline Pincer Complexes of Gold(III) Containing Semiperfluorinated Chains
- PMID: 35874197
- PMCID: PMC9301954
- DOI: 10.1021/acsomega.2c03669
Synthesis, Mesomorphism, Photophysics, and Device Properties of Liquid-Crystalline Pincer Complexes of Gold(III) Containing Semiperfluorinated Chains
Abstract
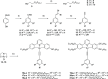
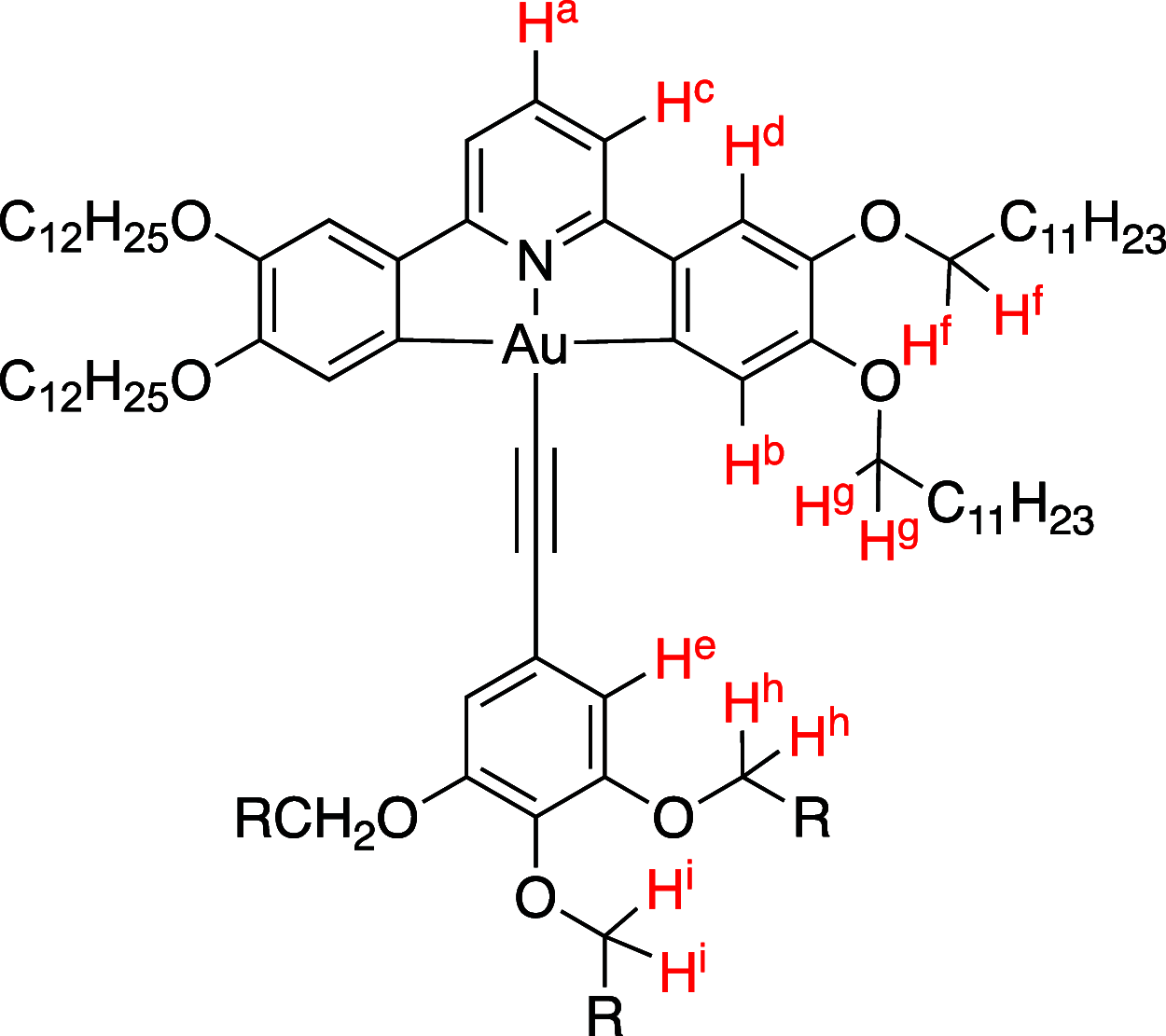
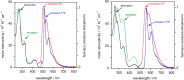
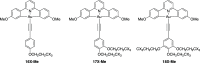
Gold(III) complexes of C∧N∧C-coordinating 2,6-diphenylpyridine pincer ligands with arylacetylide co-ligands are known triplet emitters at room temperature. We have reported previously that by functionalizing both the pincer ligand and the phenylacetylene with alkoxy chains, liquid crystallinity may be induced, with the complexes showing columnar mesophases. We now report new derivatives in which the phenylacetylene incorporates one, two, or three 1H,1H,2H,2H-perfluoroalkyl chains. In terms of intermolecular interactions, solution 1H NMR experiments suggest that the semiperfluoroalkyl chains promote a parallel, head-to-head arrangement of neighboring molecules relative to one another, rather than the anti-parallel, head-to-tail orientation found for the all-hydrocarbon materials. In terms of the liquid crystal properties, the complexes show columnar phases, with the addition of the more rigid fluorocarbon chains leading to a stabilization of both the crystal and liquid crystal mesophases. Mesophase temperature ranges were also wider. Interestingly, the amphiphilic nature of these complexes is evident through the observation of a frustrated columnar nematic phase between a Colr and a Colh phase, an observation recently reported in detail for one compound (Liq. Cryst., 2022, doi: 10.1080/02678292.2021.1991017). While calculation shows that, despite the "electronic insulation" provided by the dimethylene spacer group in the semiperfluoroalkyl chains, a small hypsochromic shift in one component of the absorption band is anticipated, experimentally this effect is not observed in the overall absorption envelope. Complexes with substituents in the 3,3',4,4'-positions of the phenyl rings of the pincer ligand once more show higher-luminescence quantum yields than the analogues with substituents in the 4,4'-positions only, associated with the lower-energy-emissive state in the former. However, in contrast to the observations with all-hydrocarbon analogues, the luminescence quantum yield of the complexes with 3,3',4,4'-substitution on the pincer increases as the number of semiperfluoroalkyl chains on the phenylacetylide increases, from 20% (one chain) to 34% (three chains). External quantum efficiencies in fabricated OLED devices are, however, low, attributed to the poor dispersion in the host materials on account of the fluorinated chains.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures









References
-
- Au V. K.-M.; Tsang D. P.-K.; Wong K. M.-C.; Chan M.-Y.; Zhu N.; Yam V. W.-W. Functionalized Bis-Cyclometalated Alkynylgold(III) Complexes: Synthesis, Characterization, Electrochemistry, Photophysics, Photochemistry, and Electroluminescence Studies. Inorg. Chem. 2013, 52, 12713–12725. 10.1021/ic4019212. - DOI - PubMed
-
- Au V. K.-M.; Tsang D. P.-K.; Wong Y.-C.; Chan M.-Y.; Yam V. W.-W. Synthesis of alkynylgold(III) complexes with bis-cyclometalating ligand derived from ethyl 2,6-diphenylisonicotinate and their structural, electrochemical, photo- and electroluminescence studies. J. Organomet. Chem. 2015, 792, 109–116. 10.1016/j.jorganchem.2015.02.037. - DOI
LinkOut - more resources
Full Text Sources

