Case Report: Dramatic Cholestasis Responsive to Steroids in a Newborn Homozygous for H63D HFE Variant
- PMID: 35874562
- PMCID: PMC9304806
- DOI: 10.3389/fped.2022.930775
Case Report: Dramatic Cholestasis Responsive to Steroids in a Newborn Homozygous for H63D HFE Variant
Abstract
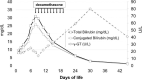
In a newborn with very precocious liver failure, cholestatic jaundice, and low γ-glutamyl transpeptidase, progressive hepatosplenomegaly induced a progressively worsening respiratory distress, that was successfully treated with steroids. Laboratory and genetic tests did not find any disease usually associated with neonatal cholestasis. However, the patient was positive for a homozygous mutation of the HFE gene, which is associated with hereditary hemochromatosis, a disease with typical onset in adulthood. Although no firm conclusions can be drawn from a single clinical case, this experience suggests that hereditary hemochromatosis could have played a role in the induction of this serious cholestasis, probably already arisen in the uterus. We suggest that hereditary hemochromatosis ought to be included in the panel of the possible causes of neonatal cholestasis and that steroids ought to be added to the pharmacological armamentarium for treating specific conditions which cause cholestasis in newborns.
Keywords: cholestasis; hereditary hemochromatosis; liver failure; newborn; steroids.
Copyright © 2022 Filippi, Tamagnini, Lorenzoni, Caciotti, Morrone and Scaramuzzo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Moyer V, Freese DK, Whitington PF, Olson AD, Brewer F, Colletti RB, et al. Guideline for the evaluation of cholestatic jaundice in infants: recommendations of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition. J Pediatr Gastroenterol Nutr. (2004) 39:115–28. 10.1097/00005176-200408000-00001 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

