The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics
- PMID: 35874608
- PMCID: PMC9298655
- DOI: 10.3389/fmolb.2022.834149
The Multi-Functional Roles of CCR7 in Human Immunology and as a Promising Therapeutic Target for Cancer Therapeutics
Abstract
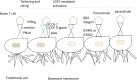
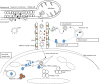
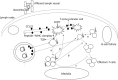
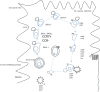
An important hallmark of the human immune system is to provide adaptive immunity against pathogens but tolerance toward self-antigens. The CC-chemokine receptor 7 (CCR7) provides a significant contribution in guiding cells to and within lymphoid organs and is important for acquiring immunity and tolerance. The CCR7 holds great importance in establishing thymic architecture and function and naïve and regulatory T-cell homing in the lymph nodes. Similarly, the receptor is a key regulator in cancer cell migration and the movement of dendritic cells. This makes the CCR7 an important receptor as a drug and prognostic marker. In this review, we discussed several biological roles of the CCR7 and its importance as a drug and prognostic marker.
Keywords: CC-chemokine receptor 7; CCL19; CCL21; drug target; immune tolerance; prognostic marker.
Copyright © 2022 Alrumaihi.
Conflict of interest statement
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
CCR7 and its ligands: balancing immunity and tolerance.Nat Rev Immunol. 2008 May;8(5):362-71. doi: 10.1038/nri2297. Nat Rev Immunol. 2008. PMID: 18379575 Review.
-
A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system.Cytokine Growth Factor Rev. 2013 Jun;24(3):269-83. doi: 10.1016/j.cytogfr.2013.03.001. Epub 2013 Apr 12. Cytokine Growth Factor Rev. 2013. PMID: 23587803 Review.
-
Fluorescently Tagged CCL19 and CCL21 to Monitor CCR7 and ACKR4 Functions.Int J Mol Sci. 2018 Dec 4;19(12):3876. doi: 10.3390/ijms19123876. Int J Mol Sci. 2018. PMID: 30518137 Free PMC article.
-
Differential expression of CCL19 by DC-Lamp+ mature dendritic cells in human lymph node versus chronically inflamed skin.J Pathol. 2003 Jan;199(1):98-106. doi: 10.1002/path.1255. J Pathol. 2003. PMID: 12474232
-
CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells.Clin Cancer Res. 2004 Apr 1;10(7):2351-8. doi: 10.1158/1078-0432.ccr-03-0195. Clin Cancer Res. 2004. PMID: 15073111
Cited by
-
Brief research report: in-depth immunophenotyping reveals stability of CD19 CAR T-cells over time.Front Immunol. 2024 Jan 22;15:1298598. doi: 10.3389/fimmu.2024.1298598. eCollection 2024. Front Immunol. 2024. PMID: 38318174 Free PMC article.
-
Integrative immunology identified interferome signatures in uveitis and systemic disease-associated uveitis.Front Immunol. 2025 Apr 9;16:1509805. doi: 10.3389/fimmu.2025.1509805. eCollection 2025. Front Immunol. 2025. PMID: 40270958 Free PMC article.
-
Role of tertiary lymphoid structures and B cells in clinical immunotherapy of gastric cancer.Front Immunol. 2025 Jan 7;15:1519034. doi: 10.3389/fimmu.2024.1519034. eCollection 2024. Front Immunol. 2025. PMID: 39840050 Free PMC article. Review.
-
Characterization of Freshly Isolated Human Peripheral Blood B Cells, Monocytes, CD4+ and CD8+ T Cells, and Skin Mast Cells by Quantitative Transcriptomics.Int J Mol Sci. 2024 Dec 4;25(23):13050. doi: 10.3390/ijms252313050. Int J Mol Sci. 2024. PMID: 39684762 Free PMC article.
-
Calcium electroporation of esophageal cancer induces gene expression changes: a sub-study of a phase I clinical trial.J Cancer Res Clin Oncol. 2023 Nov;149(17):16031-16042. doi: 10.1007/s00432-023-05357-y. Epub 2023 Sep 9. J Cancer Res Clin Oncol. 2023. PMID: 37688629 Free PMC article. Clinical Trial.
References
Publication types
LinkOut - more resources
Full Text Sources

