Dual Antigen T Cell Engagers Targeting CA9 as an Effective Immunotherapeutic Modality for Targeting CA9 in Solid Tumors
- PMID: 35874663
- PMCID: PMC9296860
- DOI: 10.3389/fimmu.2022.905768
Dual Antigen T Cell Engagers Targeting CA9 as an Effective Immunotherapeutic Modality for Targeting CA9 in Solid Tumors
Abstract
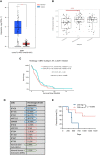
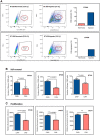
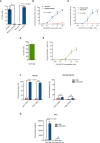
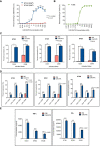
Glioblastomas (GBM), the most common malignant primary adult brain tumors, are uniformly lethal and are in need of improved therapeutic modalities. GBM contain extensive regions of hypoxia and are enriched in therapy resistant brain tumor-initiating cells (BTICs). Carbonic anhydrase 9 (CA9) is a hypoxia-induced cell surface enzyme that plays an important role in maintenance of stem cell survival and therapeutic resistance. Here we demonstrate that CA9 is highly expressed in patient-derived BTICs. CA9+ GBM BTICs showed increased self-renewal and proliferative capacity. To target CA9, we developed dual antigen T cell engagers (DATEs) that were exquisitely specific for CA9-positive patient-derived clear cell Renal Cell Carcinoma (ccRCC) and GBM cells. Combined treatment of either ccRCC or GBM cells with the CA9 DATE and T cells resulted in T cell activation, increased release of pro-inflammatory cytokines and enhanced cytotoxicity in a CA9-dependent manner. Treatment of ccRCC and GBM patient-derived xenografts markedly reduced tumor burden and extended survival. These data suggest that the CA9 DATE could provide a novel therapeutic strategy for patients with solid tumors expressing CA9 to overcome treatment resistance. .
Keywords: CA9; clear cell renal cell carcinoma; dual antigen T cell engagers; glioblastoma; hypoxic niche; immunotherapy.
Copyright © 2022 Tatari, Zhang, Chafe, McKenna, Lawson, Subapanditha, Shaikh, Seyfrid, Savage, Venugopal, Moffat and Singh.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Hypoxia induced CA9 inhibitory targeting by two different sulfonamide derivatives including acetazolamide in human glioblastoma.Bioorg Med Chem. 2013 Jul 1;21(13):3949-57. doi: 10.1016/j.bmc.2013.03.068. Epub 2013 Apr 12. Bioorg Med Chem. 2013. PMID: 23706268
-
Carbonic anhydrase-9-targeted near-infrared photoimmunotherapy as a theranostic modality for clear cell renal cell carcinoma.Int J Cancer. 2025 Jun 15;156(12):2377-2388. doi: 10.1002/ijc.35364. Epub 2025 Feb 12. Int J Cancer. 2025. PMID: 39936451 Free PMC article.
-
Carbonic anhydrase 9 in clear cell renal cell carcinoma: a marker for diagnosis, prognosis and treatment.Eur J Cancer. 2010 Dec;46(18):3141-8. doi: 10.1016/j.ejca.2010.07.020. Epub 2010 Aug 13. Eur J Cancer. 2010. PMID: 20709527 Review.
-
Modulation of carbonic anhydrase 9 (CA9) in human brain cancer.Curr Pharm Des. 2010;16(29):3288-99. doi: 10.2174/138161210793429788. Curr Pharm Des. 2010. PMID: 20819065 Review.
-
Generation of anti-tumour immune response using dendritic cells pulsed with carbonic anhydrase IX-Acinetobacter baumannii outer membrane protein A fusion proteins against renal cell carcinoma.Clin Exp Immunol. 2012 Jan;167(1):73-83. doi: 10.1111/j.1365-2249.2011.04489.x. Clin Exp Immunol. 2012. PMID: 22132887 Free PMC article.
Cited by
-
Identification of the important role of CA9 in immune infiltration and prognosis in cervical cancer.Future Sci OA. 2025 Dec;11(1):2532314. doi: 10.1080/20565623.2025.2532314. Epub 2025 Jul 14. Future Sci OA. 2025. PMID: 40657793 Free PMC article.
-
Pharmacological inhibition of hypoxia induced acidosis employing a CAIX inhibitor sensitizes gemcitabine resistant PDAC cells.Sci Rep. 2025 May 14;15(1):16782. doi: 10.1038/s41598-025-93388-5. Sci Rep. 2025. PMID: 40369181 Free PMC article.
-
Format-tuning of in vivo-launched bispecific T cell engager enhances efficacy against renal cell carcinoma.J Immunother Cancer. 2024 Jun 4;12(6):e008733. doi: 10.1136/jitc-2023-008733. J Immunother Cancer. 2024. PMID: 38834201 Free PMC article.
-
Recent advances and future perspectives of CAR-T cell therapy in head and neck cancer.Front Immunol. 2023 Jun 29;14:1213716. doi: 10.3389/fimmu.2023.1213716. eCollection 2023. Front Immunol. 2023. PMID: 37457699 Free PMC article. Review.
-
Bispecific T-Cell Engagers and Chimeric Antigen Receptor T-Cell Therapies in Glioblastoma: An Update.Curr Oncol. 2023 Sep 15;30(9):8501-8549. doi: 10.3390/curroncol30090619. Curr Oncol. 2023. PMID: 37754534 Free PMC article. Review.
References
-
- Ostrom QT, Gittleman H, de Blank PM, Finlay JL, Gurney JG, McKean-Cowdin R, et al. . American Brain Tumor Association Adolescent and Young Adult Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2008-2012. Neuro Oncol (2016) 18 Suppl 1:i1–i50. doi: 10.1093/neuonc/nov297 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

