iPSCs in NK Cell Manufacturing and NKEV Development
- PMID: 35874677
- PMCID: PMC9305199
- DOI: 10.3389/fimmu.2022.890894
iPSCs in NK Cell Manufacturing and NKEV Development
Abstract
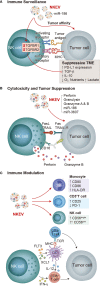
Natural killer (NK) cell immunotherapies for cancer can complement existing T cell therapies while benefiting from advancements already made in the immunotherapy field. For NK cell manufacturing, induced pluripotent stem cells (iPSCs) offer advantages including eliminating donor variation and providing an ideal platform for genome engineering. At the same time, extracellular vesicles (EVs) have become a major research interest, and purified NK cell extracellular vesicles (NKEVs) have been shown to reproduce the key functions of their parent NK cells. NKEVs have the potential to be developed into a standalone therapeutic with reduced complexity and immunogenicity compared to cell therapies. This review explores the role iPSC technology can play in both NK cell manufacturing and NKEV development.
Keywords: cancer; exosomes; extracellular vesicles; genome engineering; immunotherapy; induced pluripotent stem cells; manufacturing; natural killer cells.
Copyright © 2022 Boyd-Gibbins, Karagiannis, Hwang and Kim.
Conflict of interest statement
NBG is the CSO and a board member of THERABEST Japan, Inc. SIK is the CSO and a board member of THERABEST Co., Ltd and the co-CEO and a board member of THERABEST Japan, Inc. DWH is the CTO and a board member of THERABEST Co., Ltd. PK declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

