CAR T-Cell Therapy Predictive Response Markers in Diffuse Large B-Cell Lymphoma and Therapeutic Options After CART19 Failure
- PMID: 35874685
- PMCID: PMC9299440
- DOI: 10.3389/fimmu.2022.904497
CAR T-Cell Therapy Predictive Response Markers in Diffuse Large B-Cell Lymphoma and Therapeutic Options After CART19 Failure
Abstract
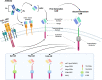
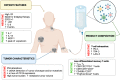
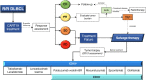
Immunotherapy with T cells genetically modified with chimeric antigen receptors (CARs) has shown significant clinical efficacy in patients with relapsed/refractory B-cell lymphoma. Nevertheless, more than 50% of treated patients do not benefit from such therapy due to either absence of response or further relapse. Elucidation of clinical and biological features that would predict clinical response to CART19 therapy is of paramount importance and eventually may allow for selection of those patients with greater chances of response. In the last 5 years, significant clinical experience has been obtained in the treatment of diffuse large B-cell lymphoma (DLBCL) patients with CAR19 T cells, and major advances have been made on the understanding of CART19 efficacy mechanisms. In this review, we discuss clinical and tumor features associated with response to CART19 in DLBCL patients as well as the impact of biological features of the infusion CART19 product on the clinical response. Prognosis of DLBCL patients that fail CART19 is poor and therapeutic approaches with new drugs are also discussed.
Keywords: CAR-T; DLBCL; antibodies; bispecific; lymphoma.
Copyright © 2022 Caballero, Escribà-Garcia, Alvarez-Fernández and Briones.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- World Health Organization . Global cancer statistics 2020: GLOBOCAN Estimates of Incidence and Mortality. Int Agency Res Cancer (2020) 68:1.
-
- Coiffier B, Thieblemont C, van den Neste E, Lepeu G, Plantier I, Castaigne S, et al. Long-Term Outcome of Patients in the LNH-98.5 Trial, the First Randomized Study Comparing Rituximab-CHOP to Standard CHOP Chemotherapy in DLBCL Patients: A Study by the Groupe D’Etudes Des Lymphomes De L’adulte. Blood (2010) 116:2040–5. doi: 10.1182/blood-2010-03-276246 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

