Small Peptide Derivatives Within the Carbohydrate Recognition Domain of SP-A2 Modulate Asthma Outcomes in Mouse Models and Human Cells
- PMID: 35874703
- PMCID: PMC9304716
- DOI: 10.3389/fimmu.2022.900022
Small Peptide Derivatives Within the Carbohydrate Recognition Domain of SP-A2 Modulate Asthma Outcomes in Mouse Models and Human Cells
Abstract
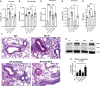
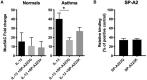
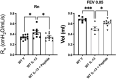
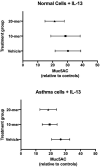
Surfactant Protein-A (SP-A) is an innate immune modulator that regulates a variety of pulmonary host defense functions. We have shown that SP-A is dysfunctional in asthma, which could be partly due to genetic heterogeneity. In mouse models and primary bronchial epithelial cells from asthmatic participants, we evaluated the functional significance of a particular single nucleotide polymorphism of SP-A2, which results in an amino acid substitution at position 223 from glutamine (Q) to lysine (K) within the carbohydrate recognition domain (CRD). We found that SP-A 223Q humanized mice had greater protection from inflammation and mucin production after IL-13 exposure as compared to SP-A-2 223K mice. Likewise, asthmatic participants with two copies the major 223Q allele demonstrated better lung function and asthma control as compared to asthmatic participants with two copies of the minor SP-A 223K allele. In primary bronchial epithelial cells from asthmatic participants, full-length recombinant SP-A 223Q was more effective at reducing IL-13-induced MUC5AC gene expression compared to SP-A 223K. Given this activity, we developed 10 and 20 amino acid peptides of SP-A2 spanning position 223Q. We show that the SP-A 223Q peptides reduce eosinophilic inflammation, mucin production and airways hyperresponsiveness in a house dust mite model of asthma, protect from lung function decline during an IL-13 challenge model in mice, and decrease IL-13-induced MUC5AC gene expression in primary airway epithelial cells from asthmatic participants. These results suggest that position 223 within the CRD of SP-A2 may modulate several outcomes relevant to asthma, and that short peptides of SP-A2 retain anti-inflammatory properties similar to that of the endogenous protein.
Keywords: SP-A; SP-A peptides; asthma; genetics; surfactant protein.
Copyright © 2022 Francisco, Wang, Marshall, Conway, Addison, Billheimer, Kimura, Numata, Chu, Voelker, Kraft and Ledford.
Conflict of interest statement
Authors JL and MK are co-founders of RaeSedo Inc, a start-up company with the goal of developing novel peptidomimetic based therapeutics derived from an active area of SP-A. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

