Pyruvate Supports RET-Dependent Mitochondrial ROS Production to Control Mycobacterium avium Infection in Human Primary Macrophages
- PMID: 35874747
- PMCID: PMC9298545
- DOI: 10.3389/fimmu.2022.891475
Pyruvate Supports RET-Dependent Mitochondrial ROS Production to Control Mycobacterium avium Infection in Human Primary Macrophages
Abstract
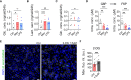
Macrophages deploy a variety of antimicrobial programs to contain mycobacterial infection. Upon activation, they undergo extensive metabolic reprogramming to meet an increase in energy demand, but also to support immune effector functions such as secretion of cytokines and antimicrobial activities. Here, we report that mitochondrial import of pyruvate is linked to production of mitochondrial ROS and control of Mycobacterium avium (M. avium) infection in human primary macrophages. Using chemical inhibition, targeted mass spectrometry and single cell image analysis, we showed that macrophages infected with M. avium switch to aerobic glycolysis without any major imbalances in the tricarboxylic acid cycle volume or changes in the energy charge. Instead, we found that pyruvate import contributes to hyperpolarization of mitochondria in infected cells and increases production of mitochondrial reactive oxygen species by the complex I via reverse electron transport, which reduces the macrophage burden of M. avium. While mycobacterial infections are extremely difficult to treat and notoriously resistant to antibiotics, this work stresses out that compounds specifically inducing mitochondrial reactive oxygen species could present themself as valuable adjunct treatments.
Keywords: Mycobacterium avium infection; glycolysis; human primary macrophages; innate immunity; mitochondrial ROS; mitochondrial pyruvate; pyruvate; reverse electron transport.
Copyright © 2022 Røst, Louet, Bruheim, Flo and Gidon.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

