Cell Membrane-Derived Vesicle: A Novel Vehicle for Cancer Immunotherapy
- PMID: 35874757
- PMCID: PMC9300949
- DOI: 10.3389/fimmu.2022.923598
Cell Membrane-Derived Vesicle: A Novel Vehicle for Cancer Immunotherapy
Abstract
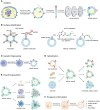
As nano-sized materials prepared by isolating, disrupting and extruding cell membranes, cellular vesicles are emerging as a novel vehicle for immunotherapeutic drugs to activate antitumor immunity. Cell membrane-derived vesicles inherit the surface characteristics and functional properties of parental cells, thus having superior biocompatibility, low immunogenicity and long circulation. Moreover, the potent antitumor effect of cellular vesicles can be achieved through surface modification, genetic engineering, hybridization, drug encapsulation, and exogenous stimulation. The capacity of cellular vesicles to combine drugs of different compositions and functions in physical space provides a promising vehicle for combinational immunotherapy of cancer. In this review, the latest advances in cellular vesicles as vehicles for combinational cancer immunotherapy are systematically summarized with focuses on manufacturing processes, cell sources, therapeutic strategies and applications, providing an insight into the potential and existing challenges of using cellular vesicles for cancer immunotherapy.
Keywords: cancer immunotherapy; cellular vesicle; combination therapy; drug delivery vehicle; drug encapsulation; membrane hybridization.
Copyright © 2022 Xu, Ju and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials

