MSC-Derived Small Extracellular Vesicles Attenuate Autoimmune Dacryoadenitis by Promoting M2 Macrophage Polarization and Inducing Tregs via miR-100-5p
- PMID: 35874782
- PMCID: PMC9298967
- DOI: 10.3389/fimmu.2022.888949
MSC-Derived Small Extracellular Vesicles Attenuate Autoimmune Dacryoadenitis by Promoting M2 Macrophage Polarization and Inducing Tregs via miR-100-5p
Abstract
Background: Mesenchymal stem cell-derived small extracellular vesicles (MSC-sEVs) have been increasingly proved as promising immunomodulators against some autoimmune disorders. However, the possible effect and the underlying mechanism of MSC-sEVs in autoimmune dry eye have been rarely studied.
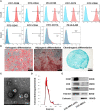
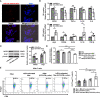
Methods: Small extracellular vesicles from human umbilical cord mesenchymal stem cells (hUC-MSC-sEVs) were subconjunctivally injected to rabbit dry eye model, and their preventive or therapeutical effects were assessed by recording the clinical and histological scores. Quantitative real-time PCR (Q-PCR), western blot and flow cytometry were performed to evaluate the immunomodulatory effects of hUC-MSC-sEVs on macrophages and T regulatory cells (Tregs) both in vivo and in vitro, and the in vitro T cell proliferation was detected by Bromodeoxyuridine (BrdU) assay. In addition, high expression of miR-100-5p in hUC-MSC-sEVs was identified by Q-PCR, and the functional role of sEVs-miR-100-5p on macrophages was explored by a series of co-culture experiments using sEVs derived from hUC-MSCs transfected with miR-100-5p inhibitor.
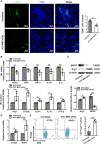
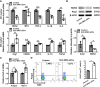
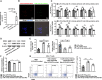
Results: We firstly demonstrated that hUC-MSC-sEVs had the preventive and therapeutical effects on rabbit autoimmune dacryoadenitis, an animal model of Sjögren's syndrome (SS) dry eye. Further investigation revealed that hUC-MSC-sEVs administration effectively elicited macrophages into an anti-inflammatory M2 phenotype and elevated the proportion of Tregs both in vivo and in vitro, which contributed to reduced inflammation and improved tissue damage. Importantly, hUC-MSC-sEVs-educated macrophages with M2-like phenotype exhibited strong capacity to inhibit CD4+ T cell proliferation and promote Treg generation in vitro. Mechanistically, miR-100-5p was highly enriched in hUC-MSC-sEVs, and knockdown of miR-100-5p in hUC-MSC-sEVs partially blunted the promotion of hUC-MSC-sEVs on M2 macrophage polarization and even attenuated the effect of hUC-MSC-sEVs-educated macrophages on T cell suppression and Treg expansion.
Conclusion: Our data indicated that hUC-MSC-sEVs alleviated autoimmune dacryoadenitis by promoting M2 macrophage polarization and Treg generation possibly through shuttling miR-100-5p. This study sheds new light on the application of MSC-sEVs as a promising therapeutic method for SS dry eye.
Keywords: autoimmune dacryoadenitis; human umbilical cord mesenchymal stem cells; macrophages; miR-100-5p; small extracellular vesicles; tregs.
Copyright © 2022 Li, Gao, Zhao, Du, Ma, Nian and Wei.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

