Single-Cell RNA Sequencing Reveals the Pathogenic Relevance of Intracranial Atherosclerosis in Blood Blister-Like Aneurysms
- PMID: 35874788
- PMCID: PMC9304558
- DOI: 10.3389/fimmu.2022.927125
Single-Cell RNA Sequencing Reveals the Pathogenic Relevance of Intracranial Atherosclerosis in Blood Blister-Like Aneurysms
Abstract
Background: Intracranial non-branching site blood blister-like aneurysms (BBA) are extremely rare and vicious. Their etiology remains elusive, and no molecular study has been carried out to reveal its pathogenic relevance to intracranial atherosclerosis. To investigate its transcriptomic landscape and underlying potential pathogenesis, we performed single-cell RNA sequencing with extensive pathological validation.
Methods: In total, 12,245 cells were recovered for single-cell RNA sequencing analysis from 1 BBA and 2 saccular intracranial aneurysms (IAs). Unbiased clustering using Seurat-based pipeline was used for cellular landscape profiling. Cellchat was used to understand intracellular communications. Furthermore, 10 BBAs and 30 IAs were retrospectively collected for pathological validations like scanning electron microscopy, H&E stain, Masson stain, Verhoeff Van Gielson stain, and immunofluorescence.
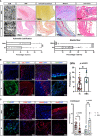
Results: Single-cell transcriptome profiled 14 total subclusters in 6 major groups, namely, 6 monocyte/macrophage clusters, 2 T&NK clusters, 3 vascular smooth muscle cell (VSMC) clusters, 1 dendritic cell, 1 B cell, and 1 endothelial cell cluster. The only mural cell identified in BBAs was VSMC-2 cluster, while mural cells in IAs comprise most clusters of VSMCs and endothelial cells. Upregulated genes in BBA-derived VSMCs are related to arterial mineralization and atherosclerosis, such as PTX3, SPP1, LOX, etc., whereas vasodilation and physiological regulatory genes such as MGP, ACTA2, and MYL9 were conversely enriched in conventional IA-derived VSMCs. Immune cells in the BBA were predominantly macrophages, with a low fraction of T&NK cells, while conventional IAs had a higher percentage of T&NK. Gene enrichment analysis suggested that macrophages in BBA were highly enriched in lipid metabolism as well as atherosclerosis. Ligand-receptor interaction suggested that secretory phosphoprotein 1 (also known as osteopontin) played a major role in mediating the intracellular communication between VSMC and macrophages, especially in BBA. Pathological experiments corroborate with the bioinformatic findings and further characterized BBAs as a thin-walled thrombotic aneurysm with severe atherosclerotic lesions, where ApoE+ macrophages and OPN+ mural cells are intimately involved in the inflammation process.
Conclusions: The preexisting intracranial atherosclerosis might predispose the parent artery to the pathogenic occurrence of BBAs. These data shed light on the pathophysiology of intracranial aneurysms and might assist in the further resolution of the complexity in aneurysm pathogenesis.
Keywords: atherosclerosis; blood blister-like aneurysm; pathogenesis; pathology; single-cell RNA sequencing.
Copyright © 2022 Wen, Wang, Chen, Li, Zheng, Fu, Zhang, Yang, You and Ma.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





References
-
- Ollikainen E, Tulamo R, Kaitainen S, Honkanen P, Lehti S, Liimatainen T, et al. . Macrophage Infiltration in the Saccular Intracranial Aneurysm Wall as a Response to Locally Lysed Erythrocytes That Promote Degeneration. J Neuropathol Exp Neurol (2018) 77(10):890–903. doi: 10.1093/jnen/nly068 - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

