Stromal and Immune Cell Dynamics in Tumor Associated Tertiary Lymphoid Structures and Anti-Tumor Immune Responses
- PMID: 35874810
- PMCID: PMC9304551
- DOI: 10.3389/fcell.2022.933113
Stromal and Immune Cell Dynamics in Tumor Associated Tertiary Lymphoid Structures and Anti-Tumor Immune Responses
Abstract
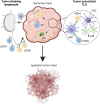
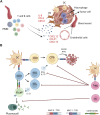
Tertiary lymphoid structures (TLS) are ectopic lymphoid organs that have been observed in chronic inflammatory conditions including cancer, where they are thought to exert a positive effect on prognosis. Both immune and non-immune cells participate in the genesis of TLS by establishing complex cross-talks requiring both soluble factors and cell-to-cell contact. Several immune cell types, including T follicular helper cells (Tfh), regulatory T cells (Tregs), and myeloid cells, may accumulate in TLS, possibly promoting or inhibiting their development. In this manuscript, we propose to review the available evidence regarding specific aspects of the TLS formation in solid cancers, including 1) the role of stromal cell composition and architecture in the recruitment of specific immune subpopulations and the formation of immune cell aggregates; 2) the contribution of the myeloid compartment (macrophages and neutrophils) to the development of antibody responses and the TLS formation; 3) the immunological and metabolic mechanisms dictating recruitment, expansion and plasticity of Tregs into T follicular regulatory cells, which are potentially sensitive to immunotherapeutic strategies directed to costimulatory receptors or checkpoint molecules.
Keywords: TLS; Tfh; Treg; neutrophils; tumor stroma.
Copyright © 2022 Rossi, Belmonte, Carnevale, Liotti, De Rosa, Jaillon, Piconese and Tripodo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures