Mitochondrial-Dependent and Independent Functions of PINK1
- PMID: 35874823
- PMCID: PMC9305176
- DOI: 10.3389/fcell.2022.954536
Mitochondrial-Dependent and Independent Functions of PINK1
Abstract
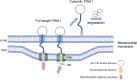
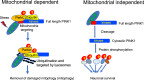
PINK1 has been characterized as a mitochondrial kinase that can target to damaged mitochondria to initiate mitophagy, a process to remove unhealthy mitochondria for protecting neuronal cells. Mutations of the human PINK1 gene are also found to cause early onset Parkinson's disease, a neurodegenerative disorder with the pathological feature of mitochondrial dysfunction. Despite compelling evidence from in vitro studies to support the role of PINK1 in regulation of mitochondrial function, there is still lack of strong in vivo evidence to validate PINK1-mediated mitophagy in the brain. In addition, growing evidence indicates that PINK1 also executes function independent of mitochondria. In this review, we discuss the mitochondrial dependent and independent functions of PINK1, aiming at elucidating how PINK1 functions differentially under different circumstances.
Keywords: PINK1; Parkinson’s disease (PD); mitochondria; mitophagy; parkin (PARK2).
Copyright © 2022 Chen, Wang, Li, Li and Yang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Akundi R. S., Huang Z., Eason J., Pandya J. D., Zhi L., Cass W. A., et al. (2011). Increased Mitochondrial Calcium Sensitivity and Abnormal Expression of Innate Immunity Genes Precede Dopaminergic Defects in Pink1-Deficient Mice. PLoS One 6 (1), e16038. 10.1371/journal.pone.0016038 - DOI - PMC - PubMed
-
- Arena G., Gelmetti V., Torosantucci L., Vignone D., Lamorte G., De Rosa P., et al. (2013). PINK1 Protects against Cell Death Induced by Mitochondrial Depolarization, by Phosphorylating Bcl-xL and Impairing its Pro-apoptotic Cleavage. Cell. Death Differ. 20 (7), 920–930. 10.1038/cdd.2013.19 - DOI - PMC - PubMed
-
- Beilina A., Van Der Brug M., Ahmad R., Kesavapany S., Miller D. W., Petsko G. A., et al. (2005). Mutations in PTEN-Induced Putative Kinase 1 Associated with Recessive Parkinsonism Have Differential Effects on Protein Stability. Proc. Natl. Acad. Sci. U.S.A. 102 (16), 5703–5708. 10.1073/pnas.0500617102 - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

