Loss of α6β4 Integrin-Mediated Hemidesmosomes Promotes Prostate Epithelial Cell Migration by Stimulating Focal Adhesion Dynamics
- PMID: 35874837
- PMCID: PMC9301336
- DOI: 10.3389/fcell.2022.886569
Loss of α6β4 Integrin-Mediated Hemidesmosomes Promotes Prostate Epithelial Cell Migration by Stimulating Focal Adhesion Dynamics
Abstract
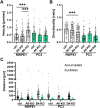
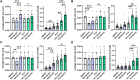
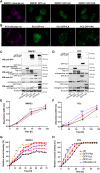
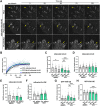
Epithelial cell adhesion is mediated by actin cytoskeleton-linked focal adhesions (FAs) and intermediate filament-associated hemidesmosomes (HDs). HDs are formed by α6β4-integrins and mediate stable anchoring to the extracellular matrix (ECM) while FAs containing β1-integrins regulate cell migration. Loss of HDs has been reported in various cancers such as prostate cancer where it correlates with increased invasive migration. Here we have studied cell migration properties and FA dynamics in genetically engineered prostate epithelial cell lines with intact or disrupted HDs. Disruption of HDs by depleting α6- or β4-integrin expression promoted collective cell migration and modulated migratory activity. Dynamic analysis of fluorescent protein-tagged FA marker proteins revealed faster FA assembly and disassembly kinetics in HD-depleted cells. FRAP analysis showed that loss of HDs correlated with faster diffusion rates of focal adhesion kinase (FAK) and vinculin in and out of FAs. These data suggest that loss of α6β4-mediated HDs promote cell migration and FA assembly dynamics by influencing the molecular diffusion rates of FAK.
Keywords: CRISPR/Cas9 knock-in; focal adhesion regulation; hemidesmosome; prostate cancer; α6β4-integrins.
Copyright © 2022 Schmidt, Kaakinen, Wenta and Manninen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

