Pitx2 Differentially Regulates the Distinct Phases of Myogenic Program and Delineates Satellite Cell Lineages During Muscle Development
- PMID: 35874842
- PMCID: PMC9298408
- DOI: 10.3389/fcell.2022.940622
Pitx2 Differentially Regulates the Distinct Phases of Myogenic Program and Delineates Satellite Cell Lineages During Muscle Development
Abstract
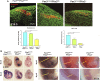
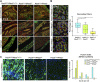
The knowledge of the molecular mechanisms that regulate embryonic myogenesis from early myogenic progenitors to myoblasts, as well as the emergence of adult satellite stem cells (SCs) during development, are key concepts to understanding the genesis and regenerative abilities of the skeletal muscle. Several previous pieces of evidence have revealed that the transcription factor Pitx2 might be a player within the molecular pathways controlling somite-derived muscle progenitors' fate and SC behavior. However, the role exerted by Pitx2 in the progression from myogenic progenitors to myoblasts including SC precursors remains unsolved. Here, we show that Pitx2 inactivation in uncommitted early myogenic precursors diminished cell proliferation and migration leading to muscle hypotrophy and a low number of SCs with decreased myogenic differentiation potential. However, the loss of Pitx2 in committed myogenic precursors gave rise to normal muscles with standard amounts of SCs exhibiting high levels of Pax7 expression. This SC population includes few MYF5+ SC-primed but increased amount of less proliferative miR-106b+cells, and display myogenic differentiation defects failing to undergo proper muscle regeneration. Overall our results demonstrate that Pitx2 is required in uncommitted myogenic progenitors but it is dispensable in committed precursors for proper myogenesis and reveal a role for this transcription factor in the generation of diverse SC subpopulations.
Keywords: Pitx2; myogenesis; myogenic precursors; satellite cells; somites.
Copyright © 2022 Ramírez de Acuña, Hernandez-Torres, Rodriguez-Outeiriño, Dominguez, Matias-Valiente, Sanchez-Fernandez, Franco and Aranega.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures








References
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

