Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2 Cells
- PMID: 35875006
- PMCID: PMC9298925
- DOI: 10.3389/fpubh.2022.906430
Genotoxicity of Particles From Grinded Plastic Items in Caco-2 and HepG2 Cells
Abstract
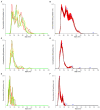
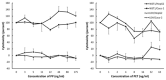
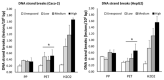
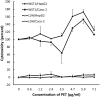
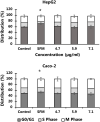
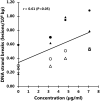
Large plastic litters degrade in the environment to micro- and nanoplastics, which may then enter the food chain and lead to human exposure by ingestion. The present study explored ways to obtain nanoplastic particles from real-life food containers. The first set of experiments gave rise to polypropylene nanoplastic suspensions with a hydrodynamic particle size range between 100 and 600 nm, whereas the same grinding process of polyethylene terephthalate (PET) produced suspensions of particles with a primary size between 100 and 300 nm. The exposure did not cause cytotoxicity measured by the lactate dehydrogenase (LDH) and water soluble tetrazolium 1 (WST-1) assays in Caco-2 and HepG2 cells. Nanoplastics of transparent PET food containers produced a modest concentration-dependent increase in DNA strand breaks, measured by the alkaline comet assay [net induction of 0.28 lesions/106 bp at the highest concentration (95% CI: 0.04; 0.51 lesions/106 base pair)]. The exposure to nanoplastics from transparent polypropylene food containers was also positively associated with DNA strand breaks [i.e., net induction of 0.10 lesions/106 base pair (95% CI: -0.04; 0.23 lesions/106 base pair)] at the highest concentration. Nanoplastics from grinding of black colored PET food containers demonstrated no effect on HepG2 and Caco-2 cells in terms of cytotoxicity, reactive oxygen species production or changes in cell cycle distribution. The net induction of DNA strand breaks was 0.43 lesions/106 bp (95% CI: 0.09; 0.78 lesions/106 bp) at the highest concentration of nanoplastics from black PET food containers. Collectively, the results indicate that exposure to nanoplastics from real-life consumer products can cause genotoxicity in cell cultures.
Keywords: DNA damage; comet assay; microplastic; nanoparticles; oxidative stress.
Copyright © 2022 Roursgaard, Hezareh Rothmann, Schulte, Karadimou, Marinelli and Møller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Nanoplastics from ground polyethylene terephthalate food containers: Genotoxicity in human lung epithelial A549 cells.Mutat Res Genet Toxicol Environ Mutagen. 2023 Nov-Dec;892:503705. doi: 10.1016/j.mrgentox.2023.503705. Epub 2023 Oct 10. Mutat Res Genet Toxicol Environ Mutagen. 2023. PMID: 37973296
-
Assessing the Release of Microplastics and Nanoplastics from Plastic Containers and Reusable Food Pouches: Implications for Human Health.Environ Sci Technol. 2023 Jul 4;57(26):9782-9792. doi: 10.1021/acs.est.3c01942. Epub 2023 Jun 21. Environ Sci Technol. 2023. PMID: 37343248
-
Exposure to nanoplastic particles and DNA damage in mammalian cells.Mutat Res Rev Mutat Res. 2023 Jul-Dec;792:108468. doi: 10.1016/j.mrrev.2023.108468. Epub 2023 Sep 4. Mutat Res Rev Mutat Res. 2023. PMID: 37666295 Review.
-
Aminated polystyrene and DNA strand breaks in A549, Caco-2, THP-1 and U937 human cell lines.Mutat Res Genet Toxicol Environ Mutagen. 2025 Apr;903:503865. doi: 10.1016/j.mrgentox.2025.503865. Epub 2025 Mar 5. Mutat Res Genet Toxicol Environ Mutagen. 2025. PMID: 40185540
-
Micro- and nanoplastic induced cellular toxicity in mammals: A review.Sci Total Environ. 2021 Feb 10;755(Pt 2):142518. doi: 10.1016/j.scitotenv.2020.142518. Epub 2020 Sep 25. Sci Total Environ. 2021. PMID: 33065507 Review.
Cited by
-
Investigating nanoplastics toxicity using advanced stem cell-based intestinal and lung in vitro models.Front Toxicol. 2023 Jan 27;5:1112212. doi: 10.3389/ftox.2023.1112212. eCollection 2023. Front Toxicol. 2023. PMID: 36777263 Free PMC article. Review.
-
Pharmaceuticals and Microplastics in Aquatic Environments: A Comprehensive Review of Pathways and Distribution, Toxicological and Ecological Effects.Int J Environ Res Public Health. 2025 May 20;22(5):799. doi: 10.3390/ijerph22050799. Int J Environ Res Public Health. 2025. PMID: 40427912 Free PMC article. Review.
-
Micro- and nanoplastics (MNPs) and their potential toxicological outcomes: State of science, knowledge gaps and research needs.NanoImpact. 2023 Oct;32:100481. doi: 10.1016/j.impact.2023.100481. Epub 2023 Sep 16. NanoImpact. 2023. PMID: 37717636 Free PMC article. Review.
-
Microfiber Emissions from Functionalized Textiles: Potential Threat for Human Health and Environmental Risks.Toxics. 2023 Apr 24;11(5):406. doi: 10.3390/toxics11050406. Toxics. 2023. PMID: 37235219 Free PMC article. Review.
-
Assessment of Ingested Micro- and Nanoplastic (MNP)-Mediated Genotoxicity in an In Vitro Model of the Small Intestinal Epithelium (SIE).Nanomaterials (Basel). 2024 May 6;14(9):807. doi: 10.3390/nano14090807. Nanomaterials (Basel). 2024. PMID: 38727401 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

