Penpulimab for Relapsed or Refractory Classical Hodgkin Lymphoma: A Multicenter, Single-Arm, Pivotal Phase I/II Trial (AK105-201)
- PMID: 35875118
- PMCID: PMC9301139
- DOI: 10.3389/fonc.2022.925236
Penpulimab for Relapsed or Refractory Classical Hodgkin Lymphoma: A Multicenter, Single-Arm, Pivotal Phase I/II Trial (AK105-201)
Abstract
Background: Nearly all anti-PD-1 antibodies are of the IgG4 isotype, and thus possess residual FcR effector functions. Such anti-PD-1 antibodies are also associated with immune tolerance and escape due to instability of the CH3 domain and Fc-Fc interaction. In this trial, we examined the efficacy and safety of penpulimab, a novel IgG1 anti-PD-1 antibody that does not bind to the Fc receptor, in patients with refractory or relapsed classical Hodgkin lymphoma (R/R cHL).
Methods: Adult patients (≥18 years of age) with R/R cHL received 200 mg penpulimab once biweekly until disease progression or unacceptable toxicities for a maximum of 24 months. The primary endpoint was objective response rate (ORR) based on the Independent Radiology Review Committee per Lugano 2014 criteria. Secondary endpoints included progression-free survival (PFS), overall survival (OS), treatment-related adverse events (TRAEs) and immune-related adverse events (irAEs).
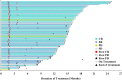
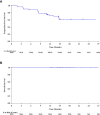
Results: A total of 94 patients were enrolled. The median follow-up was 15.8 months. The ORR was 89.4% (95% CI 80.8%, 95.0%) in the full analysis set (85 patients). Forty (47.1%) patients achieved complete remission, 36 (42.4%) patients achieved partial remission. The 12-month PFS rate was 72.1% (95% CI 60.5%, 80.8%) and the 18-month OS rate was 100%. Totally 97.9% (92/94) of patients experienced at least one TRAE. The rate of grade 3 and above TRAEs was 26.6% (25/94). In addition, 51 (54.3%) patients experienced an irAE, and 4 (4.3%) patients developed grade 3 or above irAEs. No irAE-related death occurred.
Conclusions: Penpulimab was effective and safe in patients with R/R cHL.
Keywords: IgG1 anti-PD-1 antibody; classical Hodgkin lymphoma; efficacy; penpulimab; safety.
Copyright © 2022 Song, Zhou, Jin, Qian, Hou, Fan, Li, Ding, Zhou, Li, Chen, Sun, Song, Jiang, Zhang, Liu, Yu, Hu, Zhao, Liu, Xue, Luo, He, Jin, Zhao, Li, Xia and Zhu.
Conflict of interest statement
XJ, MZ, BL and YX are employees of Akeso Biopharma Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The authors declare that this study received funding from Akeso Biopharma Co., Ltd. The funder had the following involvement with the study: sponsored this trial, and participated in the study design, data collection, statistical analysis, and provided medical writing support.
Figures

Similar articles
-
Penpulimab, an Fc-Engineered IgG1 Anti-PD-1 Antibody, With Improved Efficacy and Low Incidence of Immune-Related Adverse Events.Front Immunol. 2022 Jun 27;13:924542. doi: 10.3389/fimmu.2022.924542. eCollection 2022. Front Immunol. 2022. PMID: 35833116 Free PMC article.
-
Clinical Activity and Safety of Penpulimab (Anti-PD-1) With Anlotinib as First-Line Therapy for Unresectable Hepatocellular Carcinoma: An Open-Label, Multicenter, Phase Ib/II Trial (AK105-203).Front Oncol. 2021 Jul 13;11:684867. doi: 10.3389/fonc.2021.684867. eCollection 2021. Front Oncol. 2021. PMID: 34327136 Free PMC article.
-
Efficacy and safety of GLS-010 (zimberelimab) in patients with relapsed or refractory classical Hodgkin lymphoma: A multicenter, single-arm, phase II study.Eur J Cancer. 2022 Mar;164:117-126. doi: 10.1016/j.ejca.2021.07.021. Epub 2021 Aug 27. Eur J Cancer. 2022. PMID: 34462189 Clinical Trial.
-
Penpulimab: First Approval.Drugs. 2021 Dec;81(18):2159-2166. doi: 10.1007/s40265-021-01640-9. Drugs. 2021. PMID: 34813051 Review.
-
Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia.Drugs. 2003;63(8):803-43. doi: 10.2165/00003495-200363080-00005. Drugs. 2003. PMID: 12662126 Review.
Cited by
-
Efficacy of Immune Checkpoint Blockade and Biomarkers of Response in Lymphoma: A Narrative Review.Biomedicines. 2023 Jun 15;11(6):1720. doi: 10.3390/biomedicines11061720. Biomedicines. 2023. PMID: 37371815 Free PMC article. Review.
-
Microenvironmental Traits of Classical Hodgkin's Lymphoma in Adolescents and Their Prognostic Impact.Cancers (Basel). 2024 Dec 18;16(24):4210. doi: 10.3390/cancers16244210. Cancers (Basel). 2024. PMID: 39766109 Free PMC article.
-
Penpulimab, an anti-PD-1 antibody, for heavily pretreated metastatic nasopharyngeal carcinoma: a single-arm phase II study.Signal Transduct Target Ther. 2024 Jun 19;9(1):148. doi: 10.1038/s41392-024-01865-6. Signal Transduct Target Ther. 2024. PMID: 38890298 Free PMC article. Clinical Trial.
-
Filling the Gap: The Immune Therapeutic Armamentarium for Relapsed/Refractory Hodgkin Lymphoma.J Clin Med. 2022 Nov 6;11(21):6574. doi: 10.3390/jcm11216574. J Clin Med. 2022. PMID: 36362802 Free PMC article. Review.
-
Hot and cold tumors: Immunological features and the therapeutic strategies.MedComm (2020). 2023 Aug 26;4(5):e343. doi: 10.1002/mco2.343. eCollection 2023 Oct. MedComm (2020). 2023. PMID: 37638340 Free PMC article. Review.
References
-
- Kuruvilla J, Ramchandren R, Santoro A, Paszkiewicz-Kozik E, Gasiorowski R, Johnson NA, et al. . Pembrolizumab Versus Brentuximab Vedotin in Relapsed or Refractory Classical Hodgkin Lymphoma (KEYNOTE-204): An Interim Analysis of a Multicentre, Randomised, Open-Label, Phase 3 Study. Lancet Oncol (2021) 22:512–24. doi: 10.1016/S1470-2045(21)00005-X - DOI - PubMed
-
- Younes A, Santoro A, Shipp M, Zinzani PL, Timmerman JM, Ansell S, et al. . Nivolumab for Classical Hodgkin’s Lymphoma After Failure of Both Autologous Stem-Cell Transplantation and Brentuximab Vedotin: A Multicentre, Multicohort, Single-Arm Phase 2 Trial. Lancet Oncol (2016) 17:1283–94. doi: 10.1016/S1470-2045(16)30167-X - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Medical

