Dual Function of Par3 in Tumorigenesis
- PMID: 35875120
- PMCID: PMC9305838
- DOI: 10.3389/fonc.2022.915957
Dual Function of Par3 in Tumorigenesis
Abstract
Cell maintenance and the establishment of cell polarity involve complicated interactions among multiple protein complexes as well as the regulation of different signaling pathways. As an important cell polarity protein, Par3 is evolutionarily conserved and involved in tight junction formation as well as tumorigenesis. In this review, we aimed to explore the function of Par3 in tumorigenesis. Research has shown that Par3 exhibits dual functions in human cancers, both tumor-promoting and tumor-suppressive. Here, we focus on the activities of Par3 in different stages and types of tumors, aiming to offer a new perspective on the molecular mechanisms that regulate the functions of Par3 in tumor development. Tumor origin, tumor microenvironment, tumor type, cell density, cell-cell contact, and the synergistic effect of Par3 and other tumor-associated signaling pathways may be important reasons for the dual function of Par3. The important role of Par3 in mammalian tumorigenesis and potential signaling pathways is context dependent.
Keywords: Par3; cell polarity; dual function; tumor-promoting; tumor-suppressive.
Copyright © 2022 Lv, Xu, Yuan, Wang and Jiang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

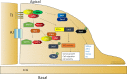
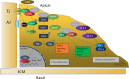

Similar articles
-
Tumor type-dependent function of the par3 polarity protein in skin tumorigenesis.Cancer Cell. 2012 Sep 11;22(3):389-403. doi: 10.1016/j.ccr.2012.08.004. Cancer Cell. 2012. PMID: 22975380
-
Dual function of partitioning-defective 3 in the regulation of YAP phosphorylation and activation.Cell Discov. 2016 Jul 5;2:16021. doi: 10.1038/celldisc.2016.21. eCollection 2016. Cell Discov. 2016. PMID: 27462467 Free PMC article.
-
The protein kinase SIK downregulates the polarity protein Par3.Oncotarget. 2017 Dec 31;9(5):5716-5735. doi: 10.18632/oncotarget.23788. eCollection 2018 Jan 19. Oncotarget. 2017. PMID: 29464029 Free PMC article.
-
Polarity complex proteins.Biochim Biophys Acta. 2008 Mar;1778(3):614-30. doi: 10.1016/j.bbamem.2007.08.029. Epub 2007 Sep 12. Biochim Biophys Acta. 2008. PMID: 18005931 Review.
-
The Par3/Par6/aPKC complex and epithelial cell polarity.Exp Cell Res. 2013 Jun 10;319(10):1357-64. doi: 10.1016/j.yexcr.2013.03.021. Epub 2013 Mar 25. Exp Cell Res. 2013. PMID: 23535009 Review.
Cited by
-
Cell polarity changes in cancer initiation and progression.J Cell Biol. 2024 Jan 1;223(1):e202308069. doi: 10.1083/jcb.202308069. Epub 2023 Dec 13. J Cell Biol. 2024. PMID: 38091012 Free PMC article. Review.
-
The polarity protein Par3 enhances renal cell carcinoma metastasis via YAP/TAZ activation.Cancer Biol Med. 2025 Jul 8;22(7):812-31. doi: 10.20892/j.issn.2095-3941.2024.0297. Cancer Biol Med. 2025. PMID: 40626835 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

