Targeting AKT-Dependent Regulation of Antioxidant Defense Sensitizes AKT-E17K Expressing Cancer Cells to Ionizing Radiation
- PMID: 35875130
- PMCID: PMC9304891
- DOI: 10.3389/fonc.2022.920017
Targeting AKT-Dependent Regulation of Antioxidant Defense Sensitizes AKT-E17K Expressing Cancer Cells to Ionizing Radiation
Abstract
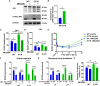
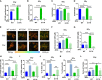
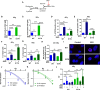
Aberrant activation of the phosphatidyl-inositol-3-kinase/protein kinase B (AKT) pathway has clinical relevance to radiation resistance, but the underlying mechanisms are incompletely understood. Protection against reactive oxygen species (ROS) plays an emerging role in the regulation of cell survival upon irradiation. AKT-dependent signaling participates in the regulation of cellular antioxidant defense. Here, we were interested to explore a yet unknown role of aberrant activation of AKT in regulating antioxidant defense in response to IR and associated radiation resistance. We combined genetic and pharmacologic approaches to study how aberrant activation of AKT impacts cell metabolism, antioxidant defense, and radiosensitivity. Therefore, we used TRAMPC1 (TrC1) prostate cancer cells overexpressing the clinically relevant AKT-variant AKT-E17K with increased AKT activity or wildtype AKT (AKT-WT) and analyzed the consequences of direct AKT inhibition (MK2206) and inhibition of AKT-dependent metabolic enzymes on the levels of cellular ROS, antioxidant capacity, metabolic state, short-term and long-term survival without and with irradiation. TrC1 cells expressing the clinically relevant AKT1-E17K variant were characterized by improved antioxidant defense compared to TrC1 AKT-WT cells and this was associated with increased radiation resistance. The underlying mechanisms involved AKT-dependent direct and indirect regulation of cellular levels of reduced glutathione (GSH). Pharmacologic inhibition of specific AKT-dependent metabolic enzymes supporting defense against oxidative stress, e.g., inhibition of glutathione synthase and glutathione reductase, improved eradication of clonogenic tumor cells, particularly of TrC1 cells overexpressing AKT-E17K. We conclude that improved capacity of TrC1 AKT-E17K cells to balance antioxidant defense with provision of energy and other metabolites upon irradiation compared to TrC1 AKT-WT cells contributes to their increased radiation resistance. Our findings on the importance of glutathione de novo synthesis and glutathione regeneration for radiation resistance of TrC1 AKT-E17K cells offer novel perspectives for improving radiosensitivity in cancer cells with aberrant AKT activity by combining IR with inhibitors targeting AKT-dependent regulation of GSH provision.
Keywords: AKT; antioxidant defense; glutathione reductase; glutathione synthase; glycolysis; hexokinase 2; radioresistance.
Copyright © 2022 Goetting, Larafa, Eul, Kunin, Jakob, Matschke and Jendrossek.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Restraining Akt1 Phosphorylation Attenuates the Repair of Radiation-Induced DNA Double-Strand Breaks and Reduces the Survival of Irradiated Cancer Cells.Int J Mol Sci. 2018 Jul 31;19(8):2233. doi: 10.3390/ijms19082233. Int J Mol Sci. 2018. PMID: 30065170 Free PMC article.
-
A gain of function mutation in AKT1 increases hexokinase 2 and diminishes oxidative stress in meningioma.Cytokine. 2024 Apr;176:156535. doi: 10.1016/j.cyto.2024.156535. Epub 2024 Feb 6. Cytokine. 2024. PMID: 38325141
-
Targeted Inhibition of Glutamine-Dependent Glutathione Metabolism Overcomes Death Resistance Induced by Chronic Cycling Hypoxia.Antioxid Redox Signal. 2016 Jul 10;25(2):89-107. doi: 10.1089/ars.2015.6589. Epub 2016 May 9. Antioxid Redox Signal. 2016. PMID: 27021152
-
A New Twist in Protein Kinase B/Akt Signaling: Role of Altered Cancer Cell Metabolism in Akt-Mediated Therapy Resistance.Int J Mol Sci. 2020 Nov 13;21(22):8563. doi: 10.3390/ijms21228563. Int J Mol Sci. 2020. PMID: 33202866 Free PMC article. Review.
-
Molecular pathways associated with oxidative stress and their potential applications in radiotherapy (Review).Int J Mol Med. 2022 May;49(5):65. doi: 10.3892/ijmm.2022.5121. Epub 2022 Mar 16. Int J Mol Med. 2022. PMID: 35293589 Free PMC article. Review.
Cited by
-
Pharmacological Akt and JNK Kinase Inhibitors 10-DEBC and SP600125 Potentiate Anti-Glioblastoma Effect of Menadione and Ascorbic Acid Combination in Human U251 Glioblastoma Cells.Biomedicines. 2023 Sep 27;11(10):2652. doi: 10.3390/biomedicines11102652. Biomedicines. 2023. PMID: 37893026 Free PMC article.
References
-
- Cantor JR, Sabatini DM. Cancer Cell Metabolism: One Hallmark, Many Faces. Cancer Discov (2012) 2(10):881–98. doi: 10.1158/2159-8290.CD-12-0345 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

