Prevalence and Outcomes of Unilateral Versus Bilateral Oophorectomy in Women With Ovarian Cancer: A Population-Based Study
- PMID: 35875152
- PMCID: PMC9304749
- DOI: 10.3389/fonc.2022.866443
Prevalence and Outcomes of Unilateral Versus Bilateral Oophorectomy in Women With Ovarian Cancer: A Population-Based Study
Abstract
Background: Unilateral oophorectomy has the benefits of preserving the ovarian function of fertility and hormone secretion, but the precise inclusion criteria for candidates for this procedure remain controversial. This study aimed to compare the prevalence and therapeutic efficiency of unilateral oophorectomy in women with ovarian cancer who underwent bilateral oophorectomy; moreover, it aimed to identify the appropriate candidates for unilateral oophorectomy.
Methods: Female patients diagnosed with stage I-III ovarian cancer between 2000 and 2017 were retrospectively identified from the Surveillance, Epidemiology, and End Results program database. Overall survival (OS) and disease-specific survival (DSS) after unilateral or bilateral (salpingo-) oophorectomy were estimated. Cumulative mortality rates (CMRs) for non-cancer comorbidities were also estimated.
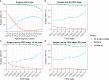
Results: A total of 28,480 women with ovarian cancer were included in this study, of whom 11,517 died during the study period. Of the patients, 7.5% and 48.0% underwent unilateral and bilateral oophorectomy, respectively. Overall, for stage-Ia tumors, unilateral oophorectomy was associated with remarkably better OS and DSS than bilateral oophorectomy (OS: p < 0.001; DSS: p = 0.01). For stage-Ib and stage-Ic ovarian tumor, there was no significant difference between the OS and DSS of patients treated by unilateral oophorectomy and those treated by bilateral oophorectomy. For stage-II and stage-III ovarian cancer, unilateral oophorectomy was associated with remarkably worse OS and DSS than bilateral oophorectomy. Among the reproductive-age women younger than 50 years, the OS and DSS of patients with stage-I tumors receiving unilateral oophorectomy were comparable to those receiving bilateral oophorectomy, even for high-grade stage-Ic tumors (all p > 0.05). For those aged 50 years and older, OS and DSS of patients with stage-I tumor receiving unilateral oophorectomy were significantly worse than those receiving bilateral oophorectomy, even for low-grade stage-Ia ovarian tumor (OS: p < 0.001; DSS: p = 0.02).
Conclusion: Unilateral oophorectomy exhibited excellent oncological superiority and was equivalent to bilateral oophorectomy for stage-I ovarian tumors among women of reproductive age. For women of reproductive age, the criteria of unilateral oophorectomy can be appropriately broadened to high-grade stage-Ic diseases because of the better performance of unilateral oophorectomy in this population.
Keywords: SEER; bilateral oophorectomy; outcomes; ovarian cancer; population-based study; prevalence; unilateral oophorectomy.
Copyright © 2022 Xiong, Zhang, Liu, Fan, Wu and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
A retrospective study of 27 ovarian tumors of low malignant potential.Eur J Gynaecol Oncol. 2002;23(5):415-8. Eur J Gynaecol Oncol. 2002. PMID: 12440814
-
Prophylactic Oophorectomy: Reducing the U.S. Death Rate from Epithelial Ovarian Cancer. A Continuing Debate.Oncologist. 1996;1(5):326-330. Oncologist. 1996. PMID: 10388011
-
Limb-salvage surgery versus extremity amputation for early-stage bone cancer in the extremities: a population-based study.Front Surg. 2023 May 31;10:1147372. doi: 10.3389/fsurg.2023.1147372. eCollection 2023. Front Surg. 2023. PMID: 37325420 Free PMC article.
-
Salpingo-oophorectomy versus cystectomy in patients with borderline ovarian tumors: a systemic review and meta-analysis on postoperative recurrence and fertility.World J Surg Oncol. 2021 Apr 21;19(1):132. doi: 10.1186/s12957-021-02241-2. World J Surg Oncol. 2021. PMID: 33882931 Free PMC article.
-
Fertility-Sparing Surgery for Ovarian Cancer.J Clin Med. 2021 Sep 18;10(18):4235. doi: 10.3390/jcm10184235. J Clin Med. 2021. PMID: 34575345 Free PMC article. Review.
References
-
- Global Burden of Disease 2019 Cancer Collaboration. Kocarnik JM, Compton K, Dean FE, Weijia F, Brian LG, et al. . Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol (2021) 8:420–44. doi: 10.1001/jamaoncol.2021.6987 - DOI - PMC - PubMed
-
- American Cancer Society . Key Statistics for Ovarian Cancer . Available at: https://www.cancer.org/cancer/ovarian-cancer/about/key-statistics.html (Accessed January 1, 2022).
-
- The SEER program . Cancer Stat Facts: Ovarian Cancer . Available at: https://seer.cancer.gov/statfacts/html/ovary.html (Accessed January 1, 2022).
-
- Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA. eds. SEER Cancer Statistics Review, 1975-2018. Bethesda, MD: National Cancer Institute; (2021). Available at: https://seer.cancer.gov/csr/1975_2018/.
-
- Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. . Global Surveillance of Cancer Survival 1995-2009: Analysis of Individual Data for 25,676,887 Patients From 279 Population-Based Registries in 67 Countries (CONCORD-2). Lancet (2015) 385(9972):977–1010. doi: 10.1016/S0140-6736(14)62038-9 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

