Low expression and Hypermethylation of ATP2B1 in Intrahepatic Cholangiocarcinoma Correlated With Cold Tumor Microenvironment
- PMID: 35875160
- PMCID: PMC9302110
- DOI: 10.3389/fonc.2022.927298
Low expression and Hypermethylation of ATP2B1 in Intrahepatic Cholangiocarcinoma Correlated With Cold Tumor Microenvironment
Abstract
Background: The efficacy of current therapeutic schedule is limited owing to fibroproliferative tumor microenvironment (TME) of cholangiocarcinoma, compelling a search for new therapeutic targets.
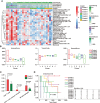
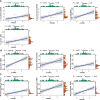
Methods: Gene expression profiles and methylation profiles were obtained from UCSC Xena. Consensus clustering was performed on the transcriptome data of cholangiocarcinoma to determine the different immune subtypes. The differentially expressed genes (DEGs) between hot tumor and cold tumors were identified. ESTIMATE was used to assess immune score, and the cases were separated into relatively superior and inferior immune score groups. Single-sample gene set enrichment analysis was applied to assess 28 immune cells in the cholangiocarcinoma microenvironment. Unsupervised consensus was applied for methylation profiling to distribute the high and low methylation groups. The correlation between DNA methylation and mRNA expression was investigated, and the relationship between the ATP2B1 gene and the immune microenvironment was explored. Finally, 77 cases of intrahepatic cholangiocarcinoma (ICC) were collected for verification.
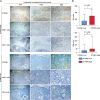
Results: Seven subtypes were related to patient outcomes (P=0.005). The proportions of CD8+ T cells in the "hot" immune type was significantly greater than that in the "cold" immune type (P<0.05). Next, DEGs and DNA methylation-governed genes were intersected, and ATP2B1 was identified as a prognosis factor in ICC (P=0.035). ATP2B1 expression was positively correlated with immune scores (P=0.005, r=0.458), the levels of infiltrating CD8+ T cells (P=0.004, r=0.47), and CD4+ T cells (P=0.027, r=0.37). Immunohistochemistry confirmed that the amounts of CD8+ and CD4+ T cells were significantly higher in ICC tissue samples than in tissues with ATP2B1 overexpression (P<0.05).
Conclusions: ATP2B1 overexpression can activate immune signals and prompt cold tumor response.
Keywords: ATP2B1; Ca2+; immune subtypes; intrahepatic cholangiocarcinoma; treatment.
Copyright © 2022 Zhang, He, Ren, Chen, Han, Qi, Chen, Luo, Zhang, Lu and Guo.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Identification of Four Immune Subtypes Characterized by Distinct Composition and Functions of Tumor Microenvironment in Intrahepatic Cholangiocarcinoma.Hepatology. 2020 Sep;72(3):965-981. doi: 10.1002/hep.31092. Epub 2020 Aug 16. Hepatology. 2020. PMID: 31875970 Free PMC article.
-
Lymphocyte-C-reactive protein ratio as a prognostic marker associated with the tumor immune microenvironment in intrahepatic cholangiocarcinoma.Int J Clin Oncol. 2021 Oct;26(10):1901-1910. doi: 10.1007/s10147-021-01962-4. Epub 2021 Jun 12. Int J Clin Oncol. 2021. PMID: 34117554
-
CTLA-4 Synergizes With PD1/PD-L1 in the Inhibitory Tumor Microenvironment of Intrahepatic Cholangiocarcinoma.Front Immunol. 2021 Aug 30;12:705378. doi: 10.3389/fimmu.2021.705378. eCollection 2021. Front Immunol. 2021. PMID: 34526987 Free PMC article.
-
The role of tumor-infiltrating lymphocytes in cholangiocarcinoma.J Exp Clin Cancer Res. 2022 Apr 7;41(1):127. doi: 10.1186/s13046-022-02340-2. J Exp Clin Cancer Res. 2022. PMID: 35392957 Free PMC article. Review.
-
Identify potential prognostic indicators and tumor-infiltrating immune cells in pancreatic adenocarcinoma.Biosci Rep. 2022 Feb 25;42(2):BSR20212523. doi: 10.1042/BSR20212523. Biosci Rep. 2022. PMID: 35083488 Free PMC article. Review.
Cited by
-
Identification of Survival-Related Genes in Acute Myeloid Leukemia (AML) Based on Cytogenetically Normal AML Samples Using Weighted Gene Coexpression Network Analysis.Dis Markers. 2022 Sep 29;2022:5423694. doi: 10.1155/2022/5423694. eCollection 2022. Dis Markers. 2022. PMID: 36212177 Free PMC article.
-
Identification and bioinformatic characterization of a serum miRNA signature for early detection of laryngeal squamous cell carcinoma.J Transl Med. 2024 Jul 10;22(1):647. doi: 10.1186/s12967-024-05385-3. J Transl Med. 2024. PMID: 38987822 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

