Race/ethnicity reporting and representation in US clinical trials: a cohort study
- PMID: 35875251
- PMCID: PMC9302767
- DOI: 10.1016/j.lana.2022.100252
Race/ethnicity reporting and representation in US clinical trials: a cohort study
Abstract
Background: Systemic progress in improving trial representation is uncertain, and previous analyses of minority trial participation have been limited to small cohorts with limited exploration of driving factors.
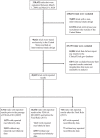
Methods: We analyzed detailed trial records from all US clinical trials registered in ClinicalTrials.gov from March 2000 to March 2020. Minority enrollment was compared to 2010 US Census demographic estimates using Wilcoxon test. We utilized logistic regression and generalized linear regression with a logit link to assess the association of possible drivers (including trials' funding source, size, phase, and design) with trials' disclosure of and amount of minority enrollment respectively.
Findings: Among 20,692 US-based trials with reported results (representing ~4·76 million enrollees), only 43% (8,871/20,692) reported any race/ethnicity data. The majority of enrollees were White (median 79·7%; interquartile range [IQR] 61·9-90·0%), followed by Black (10·0%; IQR 2·5-23·5%), Hispanic/Latino (6·0%; IQR 0·43-15·4%), Asian (1·0%; IQR 0·0-4·1%), and American Indian (0·0%; IQR 0·0-0·2%). Median combined enrollment of minority race/ethnicity groups (Black, Hispanic/Latino, Asian, American Indian, Other/Multi) was below census estimates (27·6%) (p<0·001) however increased at an annual rate of 1·7%. Industry and Academic funding were negatively associated with race/ethnicity reporting (Industry adjusted odds ratio [aOR]: 0·42, 95% confidence interval [CI]: 0·38 to 0·46, p<0.0001; Academic aOR: 0·45, CI: 0·41 to 0·50, p<0.0001). Industry also had a negative association with the proportion of minority ethnicity enrollees (aOR: 0·69, CI: 0·60 to 0·79) compared to US Government-funded trials.
Interpretation: Over the past two decades, the majority of US trials in ClinicalTrials.gov do not report race/ethnicity enrollment data, and minorities are underrepresented in trials with modest improvement over time.
Funding: Stanford Medical Scholars Research Funding, the National Heart, Lung, and Blood Institute, NIH (1K01HL144607) and the American Heart Association/Robert Wood Johnson Medical Faculty Development Program.
Conflict of interest statement
MRC has been compensated for consulting services to Pfizer in the Global Epidemiology Unit and to the Bill and Melinda Gates Foundation as a Scientific Review Lead.
Figures



References
-
- National Institute of Health (NIH) National Institute of Health (NIH); 1997. S.1 - National Institutes of Health Revitalization Act of 1993 Subtitle B-Clinical Research Equity Regarding Women and Minorities Part I-Women and Minorities as Subjects in Clinical Research Sec. 131. Requirement of Inclusion in Research.https://orwh.od.nih.gov/sites/orwh/files/docs/NIH-Revitalization-Act-199...
-
- Andrews K. Racism is the public health crisis. Lancet. 2021;397:1342–1343. (London, England) - PubMed
-
- Warren R.C., Forrow L., Hodge D.A., Truog R.D. Trustworthiness before trust — Covid-19 vaccine trials and the black community. N Engl J Med. 2020;383:e121. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

