GPR39 Deficiency Impairs Memory and Alters Oxylipins and Inflammatory Cytokines Without Affecting Cerebral Blood Flow in a High-Fat Diet Mouse Model of Cognitive Impairment
- PMID: 35875352
- PMCID: PMC9298837
- DOI: 10.3389/fncel.2022.893030
GPR39 Deficiency Impairs Memory and Alters Oxylipins and Inflammatory Cytokines Without Affecting Cerebral Blood Flow in a High-Fat Diet Mouse Model of Cognitive Impairment
Abstract
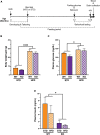
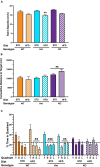
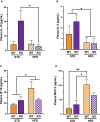
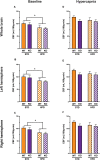
Vascular cognitive impairment (VCI) is the second most common cause of dementia. There is no treatment for VCI, in part due to a lack of understanding of the underlying mechanisms. The G-protein coupled receptor 39 (GPR39) is regulated by arachidonic acid (AA)-derived oxylipins that have been implicated in VCI. Furthermore, GPR39 is increased in microglia of post mortem human brains with VCI. Carriers of homozygous GPR39 SNPs have a higher burden of white matter hyperintensity, an MRI marker of VCI. We tested the hypothesis that GPR39 plays a protective role against high-fat diet (HFD)-induced cognitive impairment, in part mediated via oxylipins actions on cerebral blood flow (CBF) and neuroinflammation. Homozygous (KO) and heterozygous (Het) GPR39 knockout mice and wild-type (WT) littermates with and without HFD for 8 months were tested for cognitive performance using the novel object recognition (NOR) and the Morris water maze (MWM) tests, followed by CBF measurements using MRI. Brain tissue and plasma oxylipins were quantified with high-performance liquid chromatography coupled to mass spectrometry. Cytokines and chemokines were measured using a multiplex assay. KO mice, regardless of diet, swam further away from platform location in the MWM compared to WT and Het mice. In the NOR test, there were no effects of genotype or diet. Brain and plasma AA-derived oxylipins formed by 11- and 15-lipoxygenase (LOX), cyclooxygenase (COX) and non-enzymatically were increased by HFD and GPR39 deletion. Interleukin-10 (IL-10) was lower in KO mice on HFD than standard diet (STD), whereas IL-4, interferon γ-induced protein-10 (IP-10) and monocyte chemotactic protein-3 (MCP-3) were altered by diet in both WT and KO, but were not affected by genotype. Resting CBF was reduced in WT and KO mice on HFD, with no change in vasoreactivity. The deletion of GPR39 did not change CBF compared to WT mice on either STD or HFD. We conclude that GPR39 plays a role in spatial memory retention and protects against HFD-induced cognitive impairment in part by modulating inflammation and AA-derived oxylipins. The results indicate that GPR39 and oxylipin pathways play a role and may serve as therapeutic targets in VCI.
Keywords: GPR39; cerebral blood flow; dementia; high fat diet; mouse; neuroinflammation; oxylipins; vascular cognitive impairment.
Copyright © 2022 Bah, Allen, Garcia-Jaramillo, Perez, Zarnegarnia, Davis, Bloom, Magana, Choi, Bobe, Pike, Raber, Maier and Alkayed.
Conflict of interest statement
NA is co-inventor of technologies related to GPR39 that have been licensed by OHSU to Vasocardea. This potential conflict of interest has been reviewed and managed by OHSU. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
-
- Bracko O., Vinarcsik L. K., Cruz Hernandez J. C., Ruiz-Uribe N. E., Haft-Javaherian M., Falkenhain K., et al. (2020). High fat diet worsens Alzheimer’s disease-related behavioral abnormalities and neuropathology in APP/PS1 mice, but not by synergistically decreasing cerebral blood flow. Sci. Rep. 10:9884. 10.1038/s41598-020-65908-y - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

