Innate Immune Tolerance in Microglia Does Not Impact on Central Nervous System Prion Disease
- PMID: 35875357
- PMCID: PMC9302378
- DOI: 10.3389/fncel.2022.918883
Innate Immune Tolerance in Microglia Does Not Impact on Central Nervous System Prion Disease
Abstract
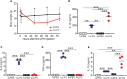
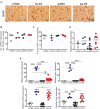
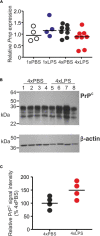
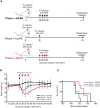
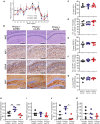
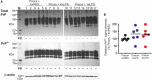
Prion diseases such as Creutzfeldt-Jakob disease in humans, bovine spongiform encephalopathy in cattle, and scrapie in sheep, are infectious and chronic neurodegenerative diseases to which there are no cures. Infection with prions in the central nervous system (CNS) ultimately causes extensive neurodegeneration, and this is accompanied by prominent microglial and astrocytic activation in affected regions. The microglia are the CNS macrophages and help maintain neuronal homeostasis, clear dead or dying cells and provide defense against pathogens. The microglia also provide neuroprotection during CNS prion disease, but their pro-inflammatory activation may exacerbate the development of the neuropathology. Innate immune tolerance induced by consecutive systemic bacterial lipopolysaccharide (LPS) treatment can induce long-term epigenetic changes in the microglia in the brain that several months later can dampen their responsiveness to subsequent LPS treatment and impede the development of neuritic damage in a transgenic mouse model of Alzheimer's disease-like pathology. We therefore reasoned that innate immune tolerance in microglia might similarly impede the subsequent development of CNS prion disease. To test this hypothesis groups of mice were first infected with prions by intracerebral injection, and 35 days later given four consecutive systemic injections with LPS to induce innate immune tolerance. Our data show that consecutive systemic LPS treatment did not affect the subsequent development of CNS prion disease. Our data suggests innate immune tolerance in microglia does not influence the subsequent onset of prion disease-induced neuropathology in mice, despite previously published evidence of this effect in an Alzheimer's disease mouse model.
Keywords: central nervous system; innate immune tolerance; lipopolysaccharide; microglia; prion disease.
Copyright © 2022 Pal, Bradford and Mabbott.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
CD11c is not required by microglia to convey neuroprotection after prion infection.PLoS One. 2023 Nov 1;18(11):e0293301. doi: 10.1371/journal.pone.0293301. eCollection 2023. PLoS One. 2023. PMID: 37910561 Free PMC article.
-
Distinctive Toll-like Receptors Gene Expression and Glial Response in Different Brain Regions of Natural Scrapie.Int J Mol Sci. 2022 Mar 25;23(7):3579. doi: 10.3390/ijms23073579. Int J Mol Sci. 2022. PMID: 35408945 Free PMC article.
-
Prion encephalopathies of animals and humans.Dev Biol Stand. 1993;80:31-44. Dev Biol Stand. 1993. PMID: 8270114 Review.
-
microRNA-146a-5p, Neurotropic Viral Infection and Prion Disease (PrD).Int J Mol Sci. 2021 Aug 25;22(17):9198. doi: 10.3390/ijms22179198. Int J Mol Sci. 2021. PMID: 34502105 Free PMC article. Review.
-
Genetic and infectious prion diseases.Arch Neurol. 1993 Nov;50(11):1129-53. doi: 10.1001/archneur.1993.00540110011002. Arch Neurol. 1993. PMID: 8105771 Review.
Cited by
-
Expression of Toll-like receptors in the cerebellum during pathogenesis of prion disease.Front Behav Neurosci. 2024 Apr 18;18:1341901. doi: 10.3389/fnbeh.2024.1341901. eCollection 2024. Front Behav Neurosci. 2024. PMID: 38698886 Free PMC article.
-
Naïve Huntington's disease microglia mount a normal response to inflammatory stimuli but display a partially impaired development of innate immune tolerance that can be counteracted by ganglioside GM1.J Neuroinflammation. 2023 Nov 23;20(1):276. doi: 10.1186/s12974-023-02963-y. J Neuroinflammation. 2023. PMID: 37996924 Free PMC article.
-
Acute LPS exposure enhances susceptibility to peripheral prion infection.Sci Rep. 2025 Mar 21;15(1):9754. doi: 10.1038/s41598-025-94003-3. Sci Rep. 2025. PMID: 40119036 Free PMC article.
-
Loss of Homeostatic Microglia Signature in Prion Diseases.Cells. 2022 Sep 21;11(19):2948. doi: 10.3390/cells11192948. Cells. 2022. PMID: 36230910 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources

