The Effects of Optogenetic Activation of Astrocytes on Spike-and-Wave Discharges in Genetic Absence Epileptic Rats
- PMID: 35875425
- PMCID: PMC9305907
- DOI: 10.1177/09727531211072423
The Effects of Optogenetic Activation of Astrocytes on Spike-and-Wave Discharges in Genetic Absence Epileptic Rats
Abstract
Background: Absence seizures (petit mal seizures) are characterized by a brief loss of consciousness without loss of postural tone. The disease is diagnosed by an electroencephalogram (EEG) showing spike-wave discharges (SWD) caused by hypersynchronous thalamocortical (TC) oscillations. There has been an explosion of research highlighting the role of astrocytes in supporting and modulating neuronal activity. Despite established in vitro evidence, astrocytes' influence on the TC network remains to be elucidated in vivo in the absence epilepsy (AE).
Purpose: In this study, we investigated the role of astrocytes in the generation and modulation of SWDs. We hypothesize that disturbances in astrocytes' function may affect the pathomechanism of AE.
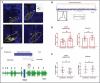
Methods: To direct the expression of channelrhodopsin-2 (ChR2) rAAV8-GFAP-ChR2(H134R)-EYFP or to control the effect of surgical intervention, AAV-CaMKIIa-EYFP was injected into the ventrobasal nucleus (VB) of the thalamus of 18 animals. After four weeks following the injection, rats were stimulated using blue light (~473 nm) and, simultaneously, the electrophysiological activity of the frontal cortical neurons was recorded for three consecutive days. The animals were then perfused, and the brain tissue was analyzed by confocal microscopy.
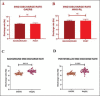
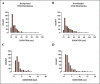
Results: A significant increase in the duration of SWD without affecting the number of SWD in genetic absence epileptic rats from Strasbourg (GAERS) compared to control injections was observed. The duration of the SWD was increased from 12.50 ± 4.41 s to 17.44 ± 6.07 following optogenetic stimulation in GAERS. The excitation of the astrocytes in Wistar Albino Glaxo Rijswijk (WAG-Rij) did not change the duration of SWD; however, stimulation resulted in a significant increase in the number of SWD from 18.52 ± 11.46 bursts/30 min to 30.17 ± 18.43 bursts/30 min. Whereas in control injection, the duration and the number of SWDs were similar at pre- and poststimulus. Both the background and poststimulus average firing rates of the SWD in WAG-Rij were significantly higher than the firing recorded in GAERS.
Conclusion: These findings suggest that VB astrocytes play a role in modulating the SWD generation in both rat models with distinct mechanisms and can present an essential target for the possible therapeutic approach for AE.
Keywords: GAERS; Optogenetics; Spike-and-wave discharges; Typical absence epilepsy; WAG-Rij.
© 2022 Indian Academy of Neurosciences (IAN).
Conflict of interest statement
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
Lipopolysaccharide induced increase in seizure activity in two animal models of absence epilepsy WAG/Rij and GAERS rats and Long Evans rats.Brain Res Bull. 2014 May;104:7-18. doi: 10.1016/j.brainresbull.2014.03.003. Epub 2014 Apr 1. Brain Res Bull. 2014. PMID: 24704320
-
Electroencephalographic differences between WAG/Rij and GAERS rat models of absence epilepsy.Epilepsy Res. 2010 May;89(2-3):185-93. doi: 10.1016/j.eplepsyres.2009.12.005. Epub 2010 Jan 25. Epilepsy Res. 2010. PMID: 20092980
-
Alterations in hippocampal and cortical densities of functionally different interneurons in rat models of absence epilepsy.Epilepsy Res. 2018 Sep;145:40-50. doi: 10.1016/j.eplepsyres.2018.05.013. Epub 2018 May 31. Epilepsy Res. 2018. PMID: 29885592
-
Spike-wave discharges in adult Sprague-Dawley rats and their implications for animal models of temporal lobe epilepsy.Epilepsy Behav. 2014 Mar;32:121-31. doi: 10.1016/j.yebeh.2014.01.004. Epub 2014 Feb 15. Epilepsy Behav. 2014. PMID: 24534480 Free PMC article. Review.
-
Genetic absence epilepsy in rats from Strasbourg--a review.J Neural Transm Suppl. 1992;35:37-69. doi: 10.1007/978-3-7091-9206-1_4. J Neural Transm Suppl. 1992. PMID: 1512594 Review.
Cited by
-
The Role of Glial Cells in the Pathophysiology of Epilepsy.Cells. 2025 Jan 10;14(2):94. doi: 10.3390/cells14020094. Cells. 2025. PMID: 39851521 Free PMC article. Review.
-
Genetic Animal Models of Idiopathic Generalized Epilepsies: What Can We Learn from Them?Biomedicines. 2025 May 26;13(6):1301. doi: 10.3390/biomedicines13061301. Biomedicines. 2025. PMID: 40564020 Free PMC article. Review.
-
Thalamocortical circuits in generalized epilepsy: Pathophysiologic mechanisms and therapeutic targets.Neurobiol Dis. 2023 Jun 1;181:106094. doi: 10.1016/j.nbd.2023.106094. Epub 2023 Mar 27. Neurobiol Dis. 2023. PMID: 36990364 Free PMC article. Review.
-
Alpha2-Adrenergic Receptors as a Pharmacological Target for Spike-Wave Epilepsy.Int J Mol Sci. 2023 Jan 12;24(2):1477. doi: 10.3390/ijms24021477. Int J Mol Sci. 2023. PMID: 36674992 Free PMC article. Review.
References
-
- Perea G, Navarrete M, and Araque A.. Tripartite synapse: Astrocytes process and control synaptic information. Trend Neurosci 2009; 32: 421–431. - PubMed
-
- Araque A, Parpura V, Sanzgiri RP, et al. Glutamate-dependent astrocyte modulation of synaptic transmission between cultured hippocampal neurons. Eur J Neurosci 1998; 10: 2129–2142. - PubMed
-
- Araque A, Sanzgiri RP, Parpura V, et al. Astrocyte-induced modulation of synaptic transmission. J Physiol Pharmacol 1999; 77: 699–706. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

