Siderophore for Lanthanide and Iron Uptake for Methylotrophy and Plant Growth Promotion in Methylobacterium aquaticum Strain 22A
- PMID: 35875576
- PMCID: PMC9301485
- DOI: 10.3389/fmicb.2022.921635
Siderophore for Lanthanide and Iron Uptake for Methylotrophy and Plant Growth Promotion in Methylobacterium aquaticum Strain 22A
Abstract
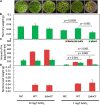
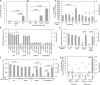
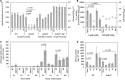
Methylobacterium and Methylorubrum species are facultative methylotrophic bacteria that are abundant in the plant phyllosphere. They have two methanol dehydrogenases, MxaF and XoxF, which are dependent on either calcium or lanthanides (Lns), respectively. Lns exist as insoluble minerals in nature, and their solubilization and uptake require a siderophore-like substance (lanthanophore). Methylobacterium species have also been identified as plant growth-promoting bacteria although the actual mechanism has not been well-investigated. This study aimed to reveal the roles of siderophore in Methylobacterium aquaticum strain 22A in Ln uptake, bacterial physiology, and plant growth promotion. The strain 22A genome contains an eight-gene cluster encoding the staphyloferrin B-like (sbn) siderophore. We demonstrate that the sbn siderophore gene cluster is necessary for growth under low iron conditions and was complemented by supplementation with citrate or spent medium of the wild type or other strains of the genera. The siderophore exhibited adaptive features, including tolerance to oxidative and nitrosative stress, biofilm formation, and heavy metal sequestration. The contribution of the siderophore to plant growth was shown by the repressive growth of duckweed treated with siderophore mutant under iron-limited conditions; however, the siderophore was dispensable for strain 22A to colonize the phyllosphere. Importantly, the siderophore mutant could not grow on methanol, but the siderophore could solubilize insoluble Ln oxide, suggesting its critical role in methylotrophy. We also identified TonB-dependent receptors (TBDRs) for the siderophore-iron complex, iron citrate, and Ln, among 12 TBDRs in strain 22A. Analysis of the siderophore synthesis gene clusters and TBDR genes in Methylobacterium genomes revealed the existence of diverse types of siderophores and TBDRs. Methylorubrum species have an exclusive TBDR for Ln uptake that has been identified as LutH. Collectively, the results of this study provide insight into the importance of the sbn siderophore in Ln chelation, bacterial physiology, and the diversity of siderophore and TBDRs in Methylobacterium species.
Keywords: Methylobacterium species; heavy metal sequestration; lanthanide; lanthanophore; plant growth promoter; siderophore.
Copyright © 2022 Juma, Fujitani, Alessa, Oyama, Yurimoto, Sakai and Tani.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources
Research Materials

