Anatomical Analysis of Transient Potential Vanilloid Receptor 1 (Trpv1+) and Mu-Opioid Receptor (Oprm1+) Co-expression in Rat Dorsal Root Ganglion Neurons
- PMID: 35875671
- PMCID: PMC9302591
- DOI: 10.3389/fnmol.2022.926596
Anatomical Analysis of Transient Potential Vanilloid Receptor 1 (Trpv1+) and Mu-Opioid Receptor (Oprm1+) Co-expression in Rat Dorsal Root Ganglion Neurons
Abstract
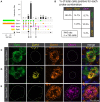
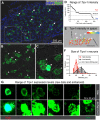
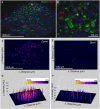
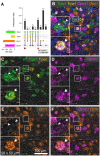
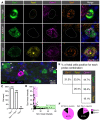
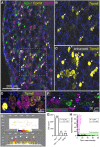
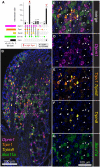
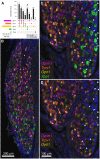
Primary afferent neurons of the dorsal root ganglia (DRG) transduce peripheral nociceptive signals and transmit them to the spinal cord. These neurons also mediate analgesic control of the nociceptive inputs, particularly through the μ-opioid receptor (encoded by Oprm1). While opioid receptors are found throughout the neuraxis and in the spinal cord tissue itself, intrathecal administration of μ-opioid agonists also acts directly on nociceptive nerve terminals in the dorsal spinal cord resulting in marked analgesia. Additionally, selective chemoaxotomy of cells expressing the TRPV1 channel, a nonselective calcium-permeable ion channel that transduces thermal and inflammatory pain, yields profound pain relief in rats, canines, and humans. However, the relationship between Oprm1 and Trpv1 expressing DRG neurons has not been precisely determined. The present study examines rat DRG neurons using high resolution multiplex fluorescent in situ hybridization to visualize molecular co-expression. Neurons positive for Trpv1 exhibited varying levels of expression for Trpv1 and co-expression of other excitatory and inhibitory ion channels or receptors. A subpopulation of densely labeled Trpv1+ neurons did not co-express Oprm1. In contrast, a population of less densely labeled Trpv1+ neurons did co-express Oprm1. This finding suggests that the medium/low Trpv1 expressing neurons represent a specific set of DRG neurons subserving the opponent processes of both transducing and inhibiting nociceptive inputs. Additionally, the medium/low Trpv1 expressing neurons co-expressed other markers implicated in pathological pain states, such as Trpa1 and Trpm8, which are involved in chemical nociception and cold allodynia, respectively, as well as Scn11a, whose mutations are implicated in familial episodic pain. Conversely, none of the Trpv1+ neurons co-expressed Spp1, which codes for osteopontin, a marker for large diameter proprioceptive neurons, validating that nociception and proprioception are governed by separate neuronal populations. Our findings support the hypothesis that the population of Trpv1 and Oprm1 coexpressing neurons may explain the remarkable efficacy of opioid drugs administered at the level of the DRG-spinal synapse, and that this subpopulation of Trpv1+ neurons is responsible for registering tissue damage.
Keywords: Kappa opioid receptor (KOR); TRPM8; dorsal root ganglia (DRG); mu opioid (MOP) receptor; nociception; opioid receptor; transient receptor potential A1 (TRPA1); transient receptor potential vanilloid-1 (TRPV1).
Copyright © 2022 Ma, Sapio, Manalo, Maric, Dougherty, Goto, Mannes and Iadarola.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
Similar articles
-
μ-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia.J Physiol. 2019 Mar;597(6):1661-1675. doi: 10.1113/JP277428. Epub 2019 Jan 16. J Physiol. 2019. PMID: 30578671 Free PMC article.
-
Loss of TRPV1-expressing sensory neurons reduces spinal mu opioid receptors but paradoxically potentiates opioid analgesia.J Neurophysiol. 2006 May;95(5):3086-96. doi: 10.1152/jn.01343.2005. Epub 2006 Feb 8. J Neurophysiol. 2006. PMID: 16467418
-
Mu-opioid receptor activation modulates transient receptor potential vanilloid 1 (TRPV1) currents in sensory neurons in a model of inflammatory pain.Mol Pharmacol. 2007 Jan;71(1):12-8. doi: 10.1124/mol.106.026740. Epub 2006 Sep 27. Mol Pharmacol. 2007. PMID: 17005903
-
The mechanism of μ-opioid receptor (MOR)-TRPV1 crosstalk in TRPV1 activation involves morphine anti-nociception, tolerance and dependence.Channels (Austin). 2015;9(5):235-43. doi: 10.1080/19336950.2015.1069450. Epub 2015 Jul 15. Channels (Austin). 2015. PMID: 26176938 Free PMC article. Review.
-
TRPV1 in dorsal root ganglion contributed to bone cancer pain.Front Pain Res (Lausanne). 2022 Nov 9;3:1022022. doi: 10.3389/fpain.2022.1022022. eCollection 2022. Front Pain Res (Lausanne). 2022. PMID: 36438444 Free PMC article. Review.
Cited by
-
Systematic identification of potential key microRNAs and circRNAs in the dorsal root ganglia of mice with sciatic nerve injury.Front Mol Neurosci. 2023 Mar 14;16:1119164. doi: 10.3389/fnmol.2023.1119164. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36998510 Free PMC article.
-
Ion channels in cancer-induced bone pain: from molecular mechanisms to clinical applications.Front Mol Neurosci. 2023 Aug 17;16:1239599. doi: 10.3389/fnmol.2023.1239599. eCollection 2023. Front Mol Neurosci. 2023. PMID: 37664239 Free PMC article. Review.
-
Efficient removal of naturally-occurring lipofuscin autofluorescence in human nervous tissue using high-intensity white light.J Pain. 2025 May;30:105359. doi: 10.1016/j.jpain.2025.105359. Epub 2025 Mar 7. J Pain. 2025. PMID: 40057214
-
The μ-opioid receptor differentiates two distinct human nociceptive populations relevant to clinical pain.Cell Rep Med. 2024 Oct 15;5(10):101788. doi: 10.1016/j.xcrm.2024.101788. Cell Rep Med. 2024. PMID: 39413733 Free PMC article.
-
Analgesic candidate adenosine A 3 receptors are expressed by perineuronal peripheral macrophages in human dorsal root ganglion and spinal cord microglia.Pain. 2024 Oct 1;165(10):2323-2343. doi: 10.1097/j.pain.0000000000003242. Epub 2024 Apr 30. Pain. 2024. PMID: 38691673
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

