Both COVID-19 infection and vaccination induce high-affinity cross-clade responses to SARS-CoV-2 variants
- PMID: 35875683
- PMCID: PMC9288251
- DOI: 10.1016/j.isci.2022.104766
Both COVID-19 infection and vaccination induce high-affinity cross-clade responses to SARS-CoV-2 variants
Abstract
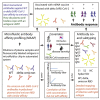
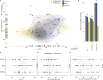
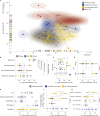
The B.1.1.529 (omicron) variant has rapidly supplanted most other SARS-CoV-2 variants. Using microfluidics-based antibody affinity profiling (MAAP), we have characterized affinity and IgG concentration in the plasma of 39 individuals with multiple trajectories of SARS-CoV-2 infection and/or vaccination. Antibody affinity was similar against the wild-type, delta, and omicron variants (K A ranges: 122 ± 155, 159 ± 148, 211 ± 307 μM-1, respectively), indicating a surprisingly broad and mature cross-clade immune response. Postinfectious and vaccinated subjects showed different IgG profiles, with IgG3 (p-value = 0.002) against spike being more prominent in the former group. Lastly, we found that the ELISA titers correlated linearly with measured concentrations (R = 0.72) but not with affinity (R = 0.29). These findings suggest that the wild-type and delta spike induce a polyclonal immune response capable of binding the omicron spike with similar affinity. Changes in titers were primarily driven by antibody concentration, suggesting that B-cell expansion, rather than affinity maturation, dominated the response after infection or vaccination.
Keywords: Disease; Immune response; Virology.
© 2022 The Author(s).
Conflict of interest statement
TPJK is a member of the board of directors of Fluidic Analytics. AA is a member of the clinical and scientific advisory board of Fluidic Analytics. AA is a member of the board of directors of Mabylon AG and AB2Bio AG. AKL, SF, SRAD, ASM, AYM, AI, and FR are employees of Fluidic Analytics. GM is a technical consultant for Fluidic Analytics. All other authors declare no competing interest.
Figures



References
-
- Crowley A.R., Natarajan H., Hederman A.P., Bobak C.A., Weiner J.A., Wieland-Alter W., Lee J., Bloch E.M., Tobian A.A.R., Redd A.D., et al. Boosting of cross-reactive antibodies to endemic coronaviruses by SARS-CoV-2 infection but not vaccination with stabilized spike. eLife. 2021;11:e75228. doi: 10.1101/2021.10.27.21265574. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

