Molecular, ecological, and behavioral drivers of the bat-virus relationship
- PMID: 35875684
- PMCID: PMC9296223
- DOI: 10.1016/j.isci.2022.104779
Molecular, ecological, and behavioral drivers of the bat-virus relationship
Abstract
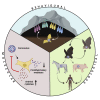
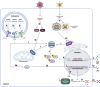
Bats perform important ecological roles in our ecosystem. However, recent studies have demonstrated that bats are reservoirs of emerging viruses that have spilled over into humans and agricultural animals to cause severe diseases. These viruses include Hendra and Nipah paramyxoviruses, Ebola and Marburg filoviruses, and coronaviruses that are closely related to severe acute respiratory syndrome coronavirus (SARS-CoV), Middle East respiratory syndrome coronavirus (MERS-CoV), and the recently emerged SARS-CoV-2. Intriguingly, bats that are naturally or experimentally infected with these viruses do not show clinical signs of disease. Here we have reviewed ecological, behavioral, and molecular factors that may influence the ability of bats to harbor viruses. We have summarized known zoonotic potential of bat-borne viruses and stress on the need for further studies to better understand the evolutionary relationship between bats and their viruses, along with discovering the intrinsic and external factors that facilitate the successful spillover of viruses from bats.
Keywords: Evolutionary biology; Virology.
© 2022 The Author(s).
Conflict of interest statement
V.G. declares no competing interests. A.B. is a co-inventor of E.fuscus kidney cell line (Efk3B) which is sold through Kerafast, Boston, USA.
Figures

Similar articles
-
Emerging viruses: Cross-species transmission of coronaviruses, filoviruses, henipaviruses, and rotaviruses from bats.Cell Rep. 2022 Jun 14;39(11):110969. doi: 10.1016/j.celrep.2022.110969. Epub 2022 May 30. Cell Rep. 2022. PMID: 35679864 Free PMC article. Review.
-
[Bats and Viruses: complex relationships].Bull Soc Pathol Exot. 2015 Oct;108(4):272-89. doi: 10.1007/s13149-015-0448-z. Epub 2015 Sep 1. Bull Soc Pathol Exot. 2015. PMID: 26330152 Free PMC article. Review.
-
Bats as reservoirs of severe emerging infectious diseases.Virus Res. 2015 Jul 2;205:1-6. doi: 10.1016/j.virusres.2015.05.006. Epub 2015 May 18. Virus Res. 2015. PMID: 25997928 Free PMC article. Review.
-
Viruses in bats and potential spillover to animals and humans.Curr Opin Virol. 2019 Feb;34:79-89. doi: 10.1016/j.coviro.2018.12.007. Epub 2019 Jan 18. Curr Opin Virol. 2019. PMID: 30665189 Free PMC article. Review.
-
A tale of endurance: bats, viruses and immune dynamics.Future Microbiol. 2024 Jun 12;19(9):841-856. doi: 10.2217/fmb-2023-0233. Epub 2024 Apr 22. Future Microbiol. 2024. PMID: 38648093 Free PMC article. Review.
Cited by
-
Bat behavioral immune responses in social contexts: current knowledge and future directions.Front Immunol. 2023 Aug 17;14:1232556. doi: 10.3389/fimmu.2023.1232556. eCollection 2023. Front Immunol. 2023. PMID: 37662931 Free PMC article. Review.
-
Bats from an area of the Colombian Caribbean reveal the circulation of Alphacoronavirus.Curr Res Parasitol Vector Borne Dis. 2025 Apr 29;7:100261. doi: 10.1016/j.crpvbd.2025.100261. eCollection 2025. Curr Res Parasitol Vector Borne Dis. 2025. PMID: 40476071 Free PMC article.
-
Differential Loss of OAS Genes Indicates Diversification of Antiviral Immunity in Mammals.Vaccines (Basel). 2023 Feb 12;11(2):419. doi: 10.3390/vaccines11020419. Vaccines (Basel). 2023. PMID: 36851296 Free PMC article.
-
Expanding the bat toolbox: Carollia perspicillata bat cell lines and reagents enable the characterization of viral susceptibility and innate immune responses.PLoS Biol. 2025 Apr 15;23(4):e3003098. doi: 10.1371/journal.pbio.3003098. eCollection 2025 Apr. PLoS Biol. 2025. PMID: 40233033 Free PMC article.
-
Viral susceptibility and innate immune competency of Carollia perspicillata bat cells produced for virological studies.bioRxiv [Preprint]. 2024 Nov 19:2024.11.19.624190. doi: 10.1101/2024.11.19.624190. bioRxiv. 2024. Update in: PLoS Biol. 2025 Apr 15;23(4):e3003098. doi: 10.1371/journal.pbio.3003098. PMID: 39605657 Free PMC article. Updated. Preprint.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

