Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction
- PMID: 35875995
- PMCID: PMC9349977
- DOI: 10.1111/jth.15830
Sustained VWF-ADAMTS-13 axis imbalance and endotheliopathy in long COVID syndrome is related to immune dysfunction
Abstract
Background: Prolonged recovery is common after acute SARS-CoV-2 infection; however, the pathophysiological mechanisms underpinning Long COVID syndrome remain unknown. VWF/ADAMTS-13 imbalance, dysregulated angiogenesis, and immunothrombosis are hallmarks of acute COVID-19. We hypothesized that VWF/ADAMTS-13 imbalance persists in convalescence together with endothelial cell (EC) activation and angiogenic disturbance. Additionally, we postulate that ongoing immune cell dysfunction may be linked to sustained EC and coagulation activation.
Patients and methods: Fifty patients were reviewed at a minimum of 6 weeks following acute COVID-19. ADAMTS-13, Weibel Palade Body (WPB) proteins, and angiogenesis-related proteins were assessed and clinical evaluation and immunophenotyping performed. Comparisons were made with healthy controls (n = 20) and acute COVID-19 patients (n = 36).
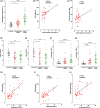
Results: ADAMTS-13 levels were reduced (p = 0.009) and the VWF-ADAMTS-13 ratio was increased in convalescence (p = 0.0004). Levels of platelet factor 4 (PF4), a putative protector of VWF, were also elevated (p = 0.0001). A non-significant increase in WPB proteins Angiopoietin-2 (Ang-2) and Osteoprotegerin (OPG) was observed in convalescent patients and WPB markers correlated with EC parameters. Enhanced expression of 21 angiogenesis-related proteins was observed in convalescent COVID-19. Finally, immunophenotyping revealed significantly elevated intermediate monocytes and activated CD4+ and CD8+ T cells in convalescence, which correlated with thrombin generation and endotheliopathy markers, respectively.
Conclusion: Our data provide insights into sustained EC activation, dysregulated angiogenesis, and VWF/ADAMTS-13 axis imbalance in convalescent COVID-19. In keeping with the pivotal role of immunothrombosis in acute COVID-19, our findings support the hypothesis that abnormal T cell and monocyte populations may be important in the context of persistent EC activation and hemostatic dysfunction during convalescence.
Keywords: Weibel Palade body exocytosis; convalescent COVID-19; endothelial cell activation; immune dysfunction; long COVID.
© 2022 The Authors. Journal of Thrombosis and Haemostasis published by Wiley Periodicals LLC on behalf of International Society on Thrombosis and Haemostasis.
Conflict of interest statement
J.S.O'D. has served on the speaker's bureau for Baxter, Bayer, Novo Nordisk, Sobi, Boehringer Ingelheim, Leo Pharma, Takeda, and Octapharma. He has also served on the advisory boards of Baxter, Sobi, Bayer, Octapharma CSL Behring, Daiichi Sankyo, Boehringer Ingelheim, Takeda, and Pfizer. J.S.O'D. has also received research grant funding awards from 3M, Baxter, Bayer, Pfizer, Shire, Takeda, and Novo Nordisk. The remaining authors have no conflicts of interest to declare.
Figures


References
-
- National Institute for Health and Care Excellence . Clinical Guidelines. COVID‐19 Rapid Guideline: Managing the Long‐Term Effects of COVID‐19. National Institute for Health and Care Excellence (NICE). Copyright © NICE 2020; 2020. - PubMed
-
- Prevention CfDCa . Post‐COVID conditions: information for healthcare providers; 2021. https://wwwcdcgov/coronavirus/2019‐ncov/hcp/clinical‐care/post‐covid‐con...
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

