Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome
- PMID: 35876011
- PMCID: PMC9327780
- DOI: 10.1080/19490976.2022.2104089
Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome
Abstract
Accumulating evidence suggests that gut microbiota as a critical mediator of gut-brain axis plays an important role in human health. Altered gut microbial profiles have been implicated in increasing the vulnerability of psychiatric disorders, such as autism, depression, and schizophrenia. However, the cellular and molecular mechanisms underlying the association remain unknown. Here, we modified the gut microbiome with antibiotics in newborn mice, and found that gut microbial alteration induced behavioral impairment by decreasing adult neurogenesis and long-term potentiation of synaptic transmission, and altering the gene expression profile in hippocampus. Reconstitution with normal gut flora produced therapeutic effects against both adult neurogenesis and behavioral deficits in the dysbiosis mice. Furthermore, our results show that circulating metabolites changes mediate the effect of gut dysbiosis on hippocampal plasticity and behavior outcomes. Elevating the serum 4-methylphenol, a small aromatic metabolite produced by gut bacteria, was found to induce autism spectrum disorder (ASD)-like behavior impairment and hippocampal dysfunction. Together our finding demonstrates that early-life gut dysbiosis and its correlated metabolites change contribute to hippocampal dysfunction and behavior impairment, hence highlight the potential microbiome-mediated therapies for treating psychiatric disorders.
Keywords: 4-methylphenol; Gut microbiota; behavior; psychiatric disorders; serum metabolites.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures
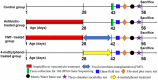
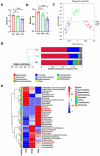
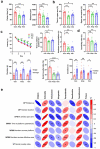
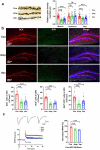
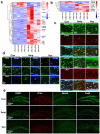
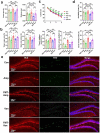


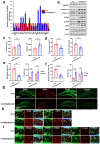
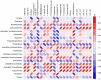
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
