Multifunctional Magnetic CuS/Gd2O3 Nanoparticles for Fluorescence/Magnetic Resonance Bimodal Imaging-Guided Photothermal-Intensified Chemodynamic Synergetic Therapy of Targeted Tumors
- PMID: 35876015
- PMCID: PMC9354791
- DOI: 10.1021/acsami.2c06503
Multifunctional Magnetic CuS/Gd2O3 Nanoparticles for Fluorescence/Magnetic Resonance Bimodal Imaging-Guided Photothermal-Intensified Chemodynamic Synergetic Therapy of Targeted Tumors
Abstract
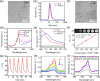
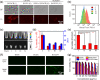
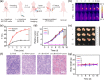
Chemodynamic therapy (CDT), which consumes endogenous hydrogen peroxide (H2O2) to generate reactive oxygen species (ROS) and causes oxidative damage to tumor cells, shows tremendous promise for advanced cancer treatment. However, the rate of ROS generation based on the Fenton reaction is prone to being restricted by inadequate H2O2 and unattainable acidity in the hypoxic tumor microenvironment. We herein report a multifunctional nanoprobe (BCGCR) integrating bimodal imaging and photothermal-enhanced CDT of the targeted tumor, which is produced by covalent conjugation of bovine serum albumin-stabilized CuS/Gd2O3 nanoparticles (NPs) with the Cy5.5 fluorophore and the tumor-targeting ligand RGD. BCGCR exhibits intense near-infrared (NIR) fluorescence and acceptable r1 relaxivity (∼15.3 mM-1 s-1) for both sensitive fluorescence imaging and high-spatial-resolution magnetic resonance imaging of tumors in living mice. Moreover, owing to the strong NIR absorbance from the internal CuS NPs, BCGCR can generate localized heat and displays a high photothermal conversion efficiency (30.3%) under 980 nm laser irradiation, which enables photothermal therapy and further intensifies ROS generation arising from the Cu-induced Fenton-like reaction for enhanced CDT. This synergetic effect shows such an excellent therapeutic efficacy that it can ablate xenografted tumors in vivo. We believe that this strategy will be beneficial to exploring other advanced nanomaterials for the clinical application of multimodal imaging-guided synergetic cancer therapies.
Keywords: copper sulfide; enhanced chemodynamic therapy; fluorescence imaging; magnetic resonance imaging; photothermal therapy.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Jin H.; Zhu T.; Huang X.; Sun M.; Li H.; Zhu X.; Liu M.; Xie Y.; Huang W.; Yan D. ROS-responsive Nanoparticles Based on Amphiphilic Hyperbranched Polyphosphoester for Drug Delivery: Light-triggered Size-reducing and Enhanced Tumor Penetration. Biomaterials 2019, 211, 68–80. 10.1016/j.biomaterials.2019.04.029. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

