In Utero Activation of Natural Killer Cells in Congenital Cytomegalovirus Infection
- PMID: 35876164
- PMCID: PMC9441208
- DOI: 10.1093/infdis/jiac307
In Utero Activation of Natural Killer Cells in Congenital Cytomegalovirus Infection
Abstract
Background: Congenital cytomegalovirus (CMV) infection is the most common infectious cause of birth defects and neurological damage in newborns. Despite a well-established role for natural killer (NK) cells in control of CMV infection in older children and adults, it remains unknown whether fetal NK cells can sense and respond to CMV infection acquired in utero.
Methods: Here, we investigate the impact of congenital CMV infection on the neonatal NK-cell repertoire by assessing the frequency, phenotype, and functional profile of NK cells in cord blood samples from newborns with congenital CMV and from uninfected controls enrolled in a birth cohort of Ugandan mothers and infants.
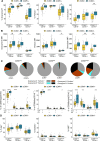
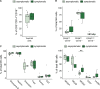
Results: We find that neonatal NK cells from congenitally CMV infected newborns show increased expression of cytotoxic mediators, signs of maturation and activation, and an expansion of mature CD56- NK cells, an NK-cell subset associated with chronic viral infections in adults. Activation was particularly prominent in NK cell subsets expressing the Fcγ receptor CD16, indicating a role for antibody-mediated immunity against CMV in utero.
Conclusions: These findings demonstrate that NK cells can be activated in utero and suggest that NK cells may be an important component of the fetal and infant immune response against CMV.
Clinical trials registration: NCT02793622.
Keywords: CD56-negative NK cells; NK cells; NKG2C; congenital CMV; cord blood; cytomegalovirus; flow cytometry; neonatal immunity.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Figures


Comment in
-
Derisking Human Cytomegalovirus Vaccine Clinical Development in Relevant Preclinical Models.J Infect Dis. 2022 Sep 4;226(4):563-565. doi: 10.1093/infdis/jiac131. J Infect Dis. 2022. PMID: 35415750 Free PMC article. No abstract available.
-
Human Cytomegalovirus Infection Primes Fetal Natural Killer Cells for Fc-Mediated Antiviral Defense.J Infect Dis. 2023 Mar 28;227(6):739-741. doi: 10.1093/infdis/jiac308. J Infect Dis. 2023. PMID: 35876548 No abstract available.
Similar articles
-
Characterization of Natural Killer Cell Profile in a Cohort of Infected Pregnant Women and Their Babies and Its Relation to CMV Transmission.Viruses. 2024 May 14;16(5):780. doi: 10.3390/v16050780. Viruses. 2024. PMID: 38793661 Free PMC article.
-
Expansion of a unique CD57⁺NKG2Chi natural killer cell subset during acute human cytomegalovirus infection.Proc Natl Acad Sci U S A. 2011 Sep 6;108(36):14725-32. doi: 10.1073/pnas.1110900108. Epub 2011 Aug 8. Proc Natl Acad Sci U S A. 2011. PMID: 21825173 Free PMC article.
-
Enumeration of NKG2C+ natural killer cells early following allogeneic stem cell transplant recipients does not allow prediction of the occurrence of cytomegalovirus DNAemia.J Med Virol. 2015 Sep;87(9):1601-7. doi: 10.1002/jmv.24198. Epub 2015 Mar 19. J Med Virol. 2015. PMID: 25802229
-
Adaptive reconfiguration of the human NK-cell compartment in response to cytomegalovirus: a different perspective of the host-pathogen interaction.Eur J Immunol. 2013 May;43(5):1133-41. doi: 10.1002/eji.201243117. Eur J Immunol. 2013. PMID: 23552990 Review.
-
About Training and Memory: NK-Cell Adaptation to Viral Infections.Adv Immunol. 2017;133:171-207. doi: 10.1016/bs.ai.2016.10.001. Epub 2016 Nov 30. Adv Immunol. 2017. PMID: 28215279 Review.
Cited by
-
Neonatal T cells unleash innate powers to combat congenital cytomegalovirus infection.J Clin Invest. 2025 Jan 2;135(1):e187789. doi: 10.1172/JCI187789. J Clin Invest. 2025. PMID: 39744950 Free PMC article.
-
Human Cytomegalovirus Immune Evasion of Natural Killer Cells: A Virus for All Seasons?Pathogens. 2025 Jun 24;14(7):629. doi: 10.3390/pathogens14070629. Pathogens. 2025. PMID: 40732677 Free PMC article. Review.
-
ADCC-activating antibodies correlate with decreased risk of congenital human cytomegalovirus transmission.JCI Insight. 2023 Jul 10;8(13):e167768. doi: 10.1172/jci.insight.167768. JCI Insight. 2023. PMID: 37427588 Free PMC article.
-
NK- and T-cell granzyme B and K expression correlates with age, CMV infection and influenza vaccine-induced antibody titres in older adults.Front Aging. 2023 Jan 5;3:1098200. doi: 10.3389/fragi.2022.1098200. eCollection 2022. Front Aging. 2023. PMID: 36685324 Free PMC article.
-
Perinatal murine cytomegalovirus infection reshapes the transcriptional profile and functionality of NK cells.Nat Commun. 2023 Oct 12;14(1):6412. doi: 10.1038/s41467-023-42182-w. Nat Commun. 2023. PMID: 37828009 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

