resPAINT: Accelerating Volumetric Super-Resolution Localisation Microscopy by Active Control of Probe Emission
- PMID: 35876263
- PMCID: PMC9804996
- DOI: 10.1002/anie.202206919
resPAINT: Accelerating Volumetric Super-Resolution Localisation Microscopy by Active Control of Probe Emission
Abstract
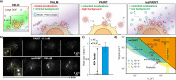
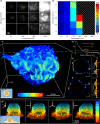
Points for accumulation in nanoscale topography (PAINT) allows practically unlimited measurements in localisation microscopy but is limited by background fluorescence at high probe concentrations, especially in volumetric imaging. We present reservoir-PAINT (resPAINT), which combines PAINT and active control of probe photophysics. In resPAINT, an activatable probe "reservoir" accumulates on target, enabling a 50-fold increase in localisation rate versus conventional PAINT, without compromising contrast. By combining resPAINT with large depth-of-field microscopy, we demonstrate super-resolution imaging of entire cell surfaces. We generalise the approach by implementing various switching strategies and 3D imaging techniques. Finally, we use resPAINT with a Fab to image membrane proteins, extending the operating regime of PAINT to include a wider range of biological interactions.
Keywords: Biophysics; Localisation Microscopy; PAINT; Single-Molecule Imaging; Super-Resolution Microscopy.
© 2022 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Thevathasan J. V., Kahnwald M., Cieśliński K., Hoess P., Peneti S. K., Reitberger M., Heid D., Kasuba K. C., Hoerner S. J., Li Y., Wu Y.-L., Mund M., Matti U., Pereira P. M., Henriques R., Nijmeijer B., Kueblbeck M., Sabinina V. J., Ellenberg J., Ries J., Nat. Methods 2019, 16, 1045–1053. - PMC - PubMed
-
- Pavani S. R. P., Piestun R., Opt. Express 2008, 16, 3484–3489. - PubMed
-
- Sims R. R., Rehman S. A., Lenz M. O., Benaissa S. I., Bruggeman E., Clark A., Sanders E. W., Ponjavic A., Ponjavic A., Ponjavic A., Muresan L., Lee S. F., O'Holleran K., Optica 2020, 7, 1065–1072.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

