Kir6.1 and SUR2B in Cantú syndrome
- PMID: 35876283
- PMCID: PMC9467476
- DOI: 10.1152/ajpcell.00154.2022
Kir6.1 and SUR2B in Cantú syndrome
Abstract
Kir6.1 and SUR2 are subunits of ATP-sensitive potassium (KATP) channels expressed in a wide range of tissues. Extensive study has implicated roles of these channel subunits in diverse physiological functions. Together they generate the predominant KATP conductance in vascular smooth muscle and are the target of vasodilatory drugs. Roles for Kir6.1/SUR2 dysfunction in disease have been suggested based on studies of animal models and human genetic discoveries. In recent years, it has become clear that gain-of-function (GoF) mutations in both genes result in Cantú syndrome (CS)-a complex, multisystem disorder. There is currently no targeted therapy for CS, but studies of mouse models of the disease reveal that pharmacological reversibility of cardiovascular and gastrointestinal pathologies can be achieved by administration of the KATP channel inhibitor, glibenclamide. Here we review the function, structure, and physiological and pathological roles of Kir6.1/SUR2B channels, with a focus on CS. Recent studies have led to much improved understanding of the underlying pathologies and the potential for treatment, but important questions remain: Can the study of genetically defined CS reveal new insights into Kir6.1/SUR2 function? Do these reveal new pathophysiological mechanisms that may be important in more common diseases? And is our pharmacological armory adequately stocked?
Keywords: ABCC9; Cantú syndrome; KCNJ8; channelopathy; smooth muscle.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures



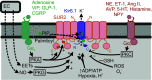

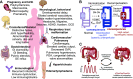
Similar articles
-
Cardiovascular consequences of KATP overactivity in Cantu syndrome.JCI Insight. 2018 Aug 9;3(15):e121153. doi: 10.1172/jci.insight.121153. eCollection 2018 Aug 9. JCI Insight. 2018. PMID: 30089727 Free PMC article.
-
Rapid Characterization of the Functional and Pharmacological Consequences of Cantú Syndrome KATP Channel Mutations in Intact Cells.J Pharmacol Exp Ther. 2023 Sep;386(3):298-309. doi: 10.1124/jpet.123.001659. Epub 2023 Aug 1. J Pharmacol Exp Ther. 2023. PMID: 37527933 Free PMC article.
-
Kir6.1-dependent KATP channels in lymphatic smooth muscle and vessel dysfunction in mice with Kir6.1 gain-of-function.J Physiol. 2020 Aug;598(15):3107-3127. doi: 10.1113/JP279612. Epub 2020 May 30. J Physiol. 2020. PMID: 32372450 Free PMC article.
-
[A new type of ATP-sensitive potassium channelopathy : Cantú syndrome].No To Hattatsu. 2016 Sep;48(5):325-31. No To Hattatsu. 2016. PMID: 30010274 Review. Japanese.
-
Adenosine Triphosphate-Sensitive Potassium Currents in Heart Disease and Cardioprotection.Card Electrophysiol Clin. 2016 Jun;8(2):323-35. doi: 10.1016/j.ccep.2016.01.005. Epub 2016 Mar 19. Card Electrophysiol Clin. 2016. PMID: 27261824 Free PMC article. Review.
Cited by
-
Automated patch clamp analysis of heterologously expressed Kir6.2/SUR1 and Kir6.1/SUR2B KATP currents.Am J Physiol Cell Physiol. 2025 Jul 1;329(1):C82-C92. doi: 10.1152/ajpcell.00266.2025. Epub 2025 Jun 4. Am J Physiol Cell Physiol. 2025. PMID: 40465479 Free PMC article.
-
Special collection on inward rectifying K+ channels.Am J Physiol Cell Physiol. 2023 Mar 1;324(3):C603-C605. doi: 10.1152/ajpcell.00457.2022. Epub 2023 Jan 23. Am J Physiol Cell Physiol. 2023. PMID: 36689674 Free PMC article.
-
Dynamic duo: Kir6 and SUR in KATP channel structure and function.Channels (Austin). 2024 Dec;18(1):2327708. doi: 10.1080/19336950.2024.2327708. Epub 2024 Mar 15. Channels (Austin). 2024. PMID: 38489043 Free PMC article. Review.
-
Novel loss-of-function variants expand ABCC9-related intellectual disability and myopathy syndrome.Brain. 2024 May 3;147(5):1822-1836. doi: 10.1093/brain/awae010. Brain. 2024. PMID: 38217872 Free PMC article.
-
Characterization of four structurally diverse inhibitors of SUR2-containing KATP channels.Channels (Austin). 2024 Dec;18(1):2398565. doi: 10.1080/19336950.2024.2398565. Epub 2024 Sep 20. Channels (Austin). 2024. PMID: 39303216 Free PMC article.
References
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

