Matrix proteoglycans in tumor inflammation and immunity
- PMID: 35876288
- PMCID: PMC9448345
- DOI: 10.1152/ajpcell.00023.2022
Matrix proteoglycans in tumor inflammation and immunity
Abstract
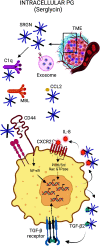
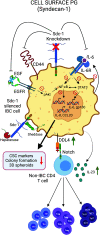
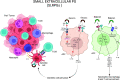
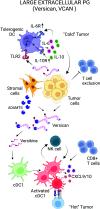
Cancer immunoediting progresses through elimination, equilibrium, and escape. Each of these phases is characterized by breaching, remodeling, and rebuilding tissue planes and structural barriers that engage extracellular matrix (ECM) components, in particular matrix proteoglycans. Some of the signals emanating from matrix proteoglycan remodeling are readily co-opted by the growing tumor to sustain an environment of tumor-promoting and immune-suppressive inflammation. Yet other matrix-derived cues can be viewed as part of a homeostatic response by the host, aiming to eliminate the tumor and restore tissue integrity. These latter signals may be harnessed for therapeutic purposes to tip the polarity of the tumor immune milieu toward anticancer immunity. In this review, we attempt to showcase the importance and complexity of matrix proteoglycan signaling in both cancer-restraining and cancer-promoting inflammation. We propose that the era of matrix diagnostics and therapeutics for cancer is fast approaching the clinic.
Keywords: dendritic; immunotherapy; matrix; proteoglycans; versican.
Conflict of interest statement
F.A. is listed as inventor on US patent US20170258898A1: “Versikine for inducing or potentiating an immune response.” None of the other authors has any conflicts of interest, financial or otherwise, to disclose.
Figures

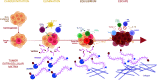
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

