Predictors of adherence to oral anticancer medications: An analysis of 2010-2018 US nationwide claims
- PMID: 35876294
- PMCID: PMC10372994
- DOI: 10.18553/jmcp.2022.28.8.831
Predictors of adherence to oral anticancer medications: An analysis of 2010-2018 US nationwide claims
Abstract
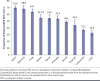
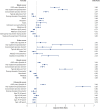
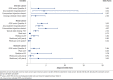
BACKGROUND: Various factors, including patient demographic and socioeconomic characteristics, patient out-of-pocket (OOP) costs, therapy-related factors, clinical characteristics, and health-system factors, can affect patient adherence to oral anticancer medications (OAMs). OBJECTIVE: To determine the proportion of patients initiating oral anticancer therapy who were adherent to OAMs and to identify significant predictors of adherence to OAMs, including patient OOP costs and patient demographics. METHODS: A retrospective cohort study was conducted using data from Optum Clinformatics Data Mart commercial claims database for 2010-2018. Patients with a new pharmacy claim for an OAM between July 1, 2010, and December 31, 2017, were followed for 6 months to ascertain their medication adherence, which was defined as a proportion of days covered value of at least 0.8. Average monthly patient OOP costs for OAM prescriptions were categorized as lower OOP costs (quartiles 1-3) and higher OOP costs (quartile 4). Separate multivariable logistic regressions were conducted to identify predictors of OAM nonadherence for each cancer type. RESULTS: Out of 37,938 patients with cancer, 51.9% were adherent to OAMs, with adherence ranging from 32.8% among those with liver cancer to 70.4% among those with brain tumor. The average monthly OOP costs of OAMs also differed by cancer type, ranging from $749 (SD = $1,014) among patients with blood cancer to $106 (SD = $439) among those with prostate cancer. Higher patient OOP costs were associated with higher odds of OAM nonadherence for many cancer types, including renal cancer (adjusted odds ratio [AOR] = 3.91; 95% CI = 2.80-5.47) and breast cancer (AOR = 1.26; 95% CI = 1.13-1.41). Additionally, patients with inpatient hospitalizations during the 6 months following OAM initiation had significantly higher odds of OAM nonadherence for all cancer types except for stomach cancer. Among patients with stomach cancer, male sex was associated with lower odds of OAM nonadherence (AOR = 0.60; 95% CI = 0.37-0.97). Among patients with renal or stomach cancer, those who had Medicare low-income subsidy had higher odds of OAM nonadherence compared with those with commercial insurance coverage. Among patients with blood cancers, Black and Hispanic patients had higher odds of OAM nonadherence compared with White patients (AOR = 1.48; 95% CI = 1.25-1.75 and AOR = 1.38; 95% CI = 1.13-1.68, respectively). CONCLUSIONS: Overall adherence to OAMs was suboptimal, and for several cancer types, adherence was worse among patients with higher OOP costs, those who were hospitalized, and those who received Medicare low-income subsidy. Policies addressing cost and access to OAMs and health-system strategies to address barriers to the effective use of OAMs are needed to improve patient access to these vital medications. DISCLOSURES: This study was funded by joint funding from the Pharmacy Quality Alliance and the National Pharmaceutical Council (NPC). Drs Vyas and Kogut were partially supported by this joint funding. Mr Descoteaux was supported by this joint funding for performing data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of PQA or NPC. Dr Campbell completed this work during his employment at Pharmacy Quality Alliance; he is now an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Conflict of interest statement
This study was funded by joint funding from the Pharmacy Quality Alliance and the National Pharmaceutical Council (NPC). Drs Vyas and Kogut were partially supported by this joint funding. Mr Descoteaux was supported by this joint funding for performing data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of PQA or NPC. Dr Campbell completed this work during his employment at Pharmacy Quality Alliance; he is now an employee of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ.
Figures

References
-
- Global Oncology Trends 2019: Therapeutics, clinical development and health system implications. The IQVIA Institute Report, May 30, 2019. Accessed December 20, 2021. https://www.iqvia.com/insights/the-iqvia-institute/reports/global-oncolo...
-
- World Health Organization. Adherence to long-term therapies: Evidence for action. Geneva, Switzerland; 2003. Accessed December 20, 2021. http://apps.who.int/iris/bitstream/handle/10665/42682/9241545992.pdf;jse...
-
- Centers for Medicare and Medicaid Services: Medicare Part D specialty tier. Accessed December 20, 2021. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrug...
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

