Systematic Review and Meta-Analysis of the Placebo Effect and its Correlates in Obsessive Compulsive Disorder
- PMID: 35876317
- PMCID: PMC10408559
- DOI: 10.1177/07067437221115029
Systematic Review and Meta-Analysis of the Placebo Effect and its Correlates in Obsessive Compulsive Disorder
Abstract
Background: Obsessive-compulsive disorder (OCD) is a major mental health condition with a lifetime prevalence rate of 1.3% among adults. While placebo effects are well described for conditions such as depressive and anxiety disorders, they have not been systematically characterized in OCD.
Objectives: We aimed to determine the impact of placebos in improving different symptom domains in patients with OCD.
Methods: We systematically searched PubMed, EMBASE, Scopus, Web of Science, Ovid, the Cochrane Library, and Google Scholar databases/search engine from inception to January 2021 for randomized controlled trials of treatments for OCD with a placebo arm. A modified Cohen's effect size (ES) was calculated using change in baseline to endpoint scores for different measurement scales within placebo arms to estimate placebo effects and to investigate their correlates by random-effects model meta-analyses.
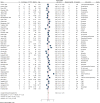
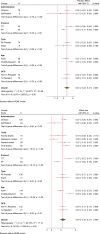
Results: Forty-nine clinical trials (placebo group n = 1993), reporting 80 OCD specific (153 measures in general) were included in the analysis. Overall placebo ES (95% confidence interval [CI]) was 0.32 (0.22-0.41) on OCD symptoms, with substantial heterogeneity (I-square = 96.1%). Among secondary outcomes, general scales, ES: 0.27 (95%CI: 0.14-0.41), demonstrated higher ES than anxiety and depression scales, ES: 0.14 (95%CI: -0.4 to 0.32) and 0.05 (95%CI: -0.05 to 0.14), respectively. Clinician-rated scales, ES: 0.27(95%CI: 0.20-0.34), had a higher ES than self-reported scales, ES: 0.07 (95%CI: -0.08 to 0.22). More recent publication year, larger placebo group sample size, shorter follow-up duration, and younger age of participants were all associated with larger placebo ES. Egger's test reflected possible small-study effect publication bias (P = 0.029).
Conclusion: Placebo effects are modest in OCD trials and are larger in clinician ratings, for younger patients, and early in the treatment course. These findings underscore the need for clinicians and scientists to be mindful of placebo effects when formulating treatments or research trials for OCD.
Systematic review registration number: PROSPERO CRD42019125979.
Contexte: Le trouble obsessionnel-compulsif (TOC) est un grand problème de santé mentale dont le taux de prévalence de durée de vie est de 1,3% chez les adultes. Bien que les effets des placebos soient bien décrits pour des conditions comme les troubles dépressifs et anxieux, ils n’ont pas été systématiquement caractérisés dans le TOC.
Objectifs: Nous visions à déterminer l’effet des placebos pour améliorer différents domaines de symptômes chez des patients souffrant du TOC.
Méthodes: Nous avons systématiquement cherché dans les bases de données et moteurs de recherche PubMed, EMBASE, Scopus, Web of Science, Ovid, the Cochrane Library, et Google Scholar du début à janvier 2021 des essais randomisés contrôlés de traitements du TOC avec un bras placebo. La taille de l’effet (TE) de Cohen modifiée a été calculée à l’aide du changement au départ vers les scores de points finaux pour différentes échelles de mesure au sein des bras placebos afin d’estimer les effets placebos et d’investiguer leurs corrélats par des méta-analyses d’un modèle à effets aléatoires.
Résultats: Quarante-neuf essais cliniques (groupe placebo n = 1993), déclarant 80 cas spécifiques du TOC (153 mesures en général) ont été inclus dans l’analyse. En général, la TE du placebo (intervalle de confiance IC à 95% était 0,32 (0,22 à 0,41) sur les symptômes du TOC, avec une hétérogénéité substantielle (I-carré = 96,1%). Parmi les résultats secondaires, les échelles générales, TE : 0,27 (IC à 95% 0,14 à 0,41), démontraient une TE plus élevée que les échelles d’anxiété et de dépression, TE : 0,14 (IC à 95% −0,4 à −0,32) et 0,05 (IC à 95% −0,05 à −0,14), respectivement. Les échelles d’évaluation clinique, TE : 0,27 (IC à 95% 0,20 à 0,34) avaient une TE plus élevée que les échelles auto-rapportées. TE : 0,07 (IC à 95% −0,08 à −0,22). L’année de publication plus récente, la plus grande taille de l’échantillon du groupe placebo, la durée plus courte du suivi, et l’âge plus jeune des participants étaient tous associés à une plus grande TE du placebo. Le test d’Egger a reflété un possible biais de publication d’effet d’étude modeste (P = 0,029).
Conclusion: Les effets placebos sont modestes dans les essais du TOC et sont plus élevés dans les évaluations des cliniciens, pour les patients plus jeunes, et tôt dans le cours du traitement. Ces résultats soulignent le besoin pour les cliniciens et les scientifiques de tenir compte des effets placebos quand ils formulent des traitements et/ou des essais de recherche pour le TOC.
Keywords: OCD; Obsessive compulsive disorder; meta-analysis; placebo; placebo effect; systematic review.
Conflict of interest statement
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. M. Sinyor receives salary support from Academic Scholars Awards from the Departments of Psychiatry at Sunnybrook Health Sciences Centre and the University of Toronto.
Figures



Similar articles
-
Control interventions in randomised trials among people with mental health disorders.Cochrane Database Syst Rev. 2022 Apr 4;4(4):MR000050. doi: 10.1002/14651858.MR000050.pub2. Cochrane Database Syst Rev. 2022. PMID: 35377466 Free PMC article.
-
Psychological and/or educational interventions for the prevention of depression in children and adolescents.Cochrane Database Syst Rev. 2004;(1):CD003380. doi: 10.1002/14651858.CD003380.pub2. Cochrane Database Syst Rev. 2004. Update in: Cochrane Database Syst Rev. 2011 Dec 07;(12):CD003380. doi: 10.1002/14651858.CD003380.pub3. PMID: 14974014 Updated.
-
Antidepressants for depression in adults with HIV infection.Cochrane Database Syst Rev. 2018 Jan 22;1(1):CD008525. doi: 10.1002/14651858.CD008525.pub3. Cochrane Database Syst Rev. 2018. PMID: 29355886 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
E-Health interventions for anxiety and depression in children and adolescents with long-term physical conditions.Cochrane Database Syst Rev. 2018 Aug 15;8(8):CD012489. doi: 10.1002/14651858.CD012489.pub2. Cochrane Database Syst Rev. 2018. PMID: 30110718 Free PMC article.
Cited by
-
Treating refractory obsessive compulsive disorder with cathodal transcranial direct current stimulation over the supplementary motor area: a large multisite randomized sham-controlled double-blind study.Front Psychiatry. 2024 May 17;15:1338594. doi: 10.3389/fpsyt.2024.1338594. eCollection 2024. Front Psychiatry. 2024. PMID: 38827437 Free PMC article.
-
A randomized crossover trial with experience sampling to test placebo effects on pathological skin-picking.Sci Rep. 2025 Jul 2;15(1):22782. doi: 10.1038/s41598-025-04360-2. Sci Rep. 2025. PMID: 40592945 Free PMC article. Clinical Trial.
-
Prevalence of co-morbid anxiety and depression in pregnancy and postpartum: a systematic review and meta-analysis.Psychol Med. 2025 Mar 13;55:e84. doi: 10.1017/S0033291725000601. Psychol Med. 2025. PMID: 40079080 Free PMC article.
-
Placebo effects in randomized trials of pharmacological and neurostimulation interventions for mental disorders: An umbrella review.Mol Psychiatry. 2024 Dec;29(12):3915-3925. doi: 10.1038/s41380-024-02638-x. Epub 2024 Jun 24. Mol Psychiatry. 2024. PMID: 38914807 Free PMC article.
-
Responsive deep brain stimulation guided by ventral striatal electrophysiology of obsession durably ameliorates compulsion.Neuron. 2024 Jan 3;112(1):73-83.e4. doi: 10.1016/j.neuron.2023.09.034. Epub 2023 Oct 20. Neuron. 2024. PMID: 37865084 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

