Quantitative Assessment of the Apical and Basolateral Membrane Expression of VEGFR2 and NRP2 in VEGF-A-stimulated Cultured Human Umbilical Vein Endothelial Cells
- PMID: 35876388
- PMCID: PMC9393510
- DOI: 10.1369/00221554221115767
Quantitative Assessment of the Apical and Basolateral Membrane Expression of VEGFR2 and NRP2 in VEGF-A-stimulated Cultured Human Umbilical Vein Endothelial Cells
Abstract
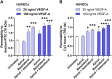
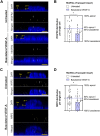
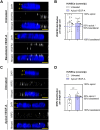
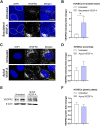
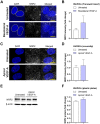
Endothelial cells (ECs) form a precisely regulated polarized monolayer in capillary walls. Vascular endothelial growth factor-A (VEGF-A) induces endothelial hyperpermeability, and VEGF-A applied to the basolateral side, but not the apical side, has been shown to be a strong barrier disruptor in blood-retinal barrier ECs. We show here that VEGF-A presented to the basolateral side of human umbilical vein ECs (HUVECs) induces higher permeability than apical stimulation, which is similar to results obtained with bovine retinal ECs. We investigated with immunocytochemistry and confocal imaging the distribution of VEGF receptor-2 (VEGFR2) and neuropilin-2 (NRP2) in perinuclear apical and basolateral membrane domains. Orthogonal z-sections of cultured HUVECs were obtained, and the fluorescence intensity at the apical and basolateral membrane compartments was measured. We found that VEGFR2 and NRP2 are evenly distributed throughout perinuclear apical and basolateral membrane compartments in unstimulated HUVECs grown on Transwell inserts, whereas basolateral VEGF-A stimulation induces a shift toward basolateral VEGFR2 and NRP2 localization. When HUVECs were grown on coverslips, the distribution of VEGFR2 and NRP2 across the perinuclear apical and basolateral membrane domains was different. Our findings demonstrate that HUVECs dynamically regulate VEGFR2 and NRP2 localization on membrane microdomains, depending on growth conditions and the polarity of VEGF-A stimulation.
Keywords: apicobasal; endothelial barrier; membrane microdomains; receptors; vascular endothelial growth factor A; vascular endothelial growth factor receptor.
Conflict of interest statement
Figures

References
-
- Witmer AN, Vrensen GFJM, van Noorden CJF, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22(1):1–29. - PubMed
-
- Klaassen I, van Noorden CJF, Schlingemann RO. Molecular basis of the inner blood-retinal barrier and its breakdown in diabetic macular edema and other pathological conditions. Prog Retin Eye Res. 2013;34:19–48. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

