Prostatic Urethral Lift for Obstructive Median Lobes: Consistent Results Across Controlled Trial and Real-World Settings
- PMID: 35876440
- PMCID: PMC9810349
- DOI: 10.1089/end.2022.0324
Prostatic Urethral Lift for Obstructive Median Lobes: Consistent Results Across Controlled Trial and Real-World Settings
Abstract
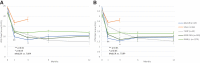
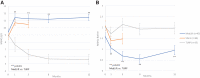
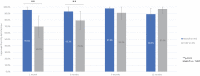
Introduction: The evidence for prostatic urethral lift (PUL), in treating lower urinary tract symptoms/benign prostatic hyperplasia (BPH) in men with obstructive median lobes (OMLs), has grown. In this study, we present the first detailed comparison of outcomes between OML patients treated with PUL in controlled and real-world settings to relevant comparators (subjects treated with transurethral resection of the prostate [TURP] and sham in randomized controlled trials [RCTs]) to demonstrate similar symptom, safety, and patient experience outcomes. Materials and Methods: Symptom and safety outcomes and patient satisfaction were compared through 12 months among controlled PUL studies: BPH6 RCT (35 men randomized to TURP); L.I.F.T. pivotal RCT in subjects with lateral lobe obstruction (66 subjects randomized to sham) and MedLift, an U.S. Food and Drug Administration-approved Investigational Device Exemption (IDE) extension of the L.I.F.T. trial (45 men with OML). Symptom improvement, catheterization, and adverse event rates were compared between MedLift subjects and OML patients (n = 187) from the large real-world retrospective (RWR) study of PUL filtered on baseline characteristics to approximate the MedLift population. Results: Posttreatment, International Prostate Symptoms Score (IPSS) improvement for MedLift subjects was 170% greater compared with sham at 3 months with significantly better quality of life (QoL), Qmax, and benign prostatic hyperplasia impact index (BPHII). Compared with TURP, MedLift IPSS and QoL improved significantly better at 1 and 3 months and with superior ejaculatory function scores at all time points after PUL. IPSS, QoL, postvoid residual (PVR), and Qmax outcomes were equivalent between MedLift and RWR OML groups at 3, 6, and 12 months. RWR OML patients did not experience higher rates of overall adverse events compared with MedLift. Conclusion: Controlled and real-world outcomes confirm PUL is a safe and effective treatment for BPH patients with and without OML.
Keywords: CCT; IPSS; benign prostatic hyperplasia; clinically controlled trials; lower urinary tract symptoms; minimally invasive surgical therapy; prostatic urethral lift; randomized controlled trials; real world; retrospective study; symptom score; transurethral resection of the prostate.
Conflict of interest statement
Drs. Eure, Roehrborn, and Rukstalis are NeoTract, Inc./Teleflex consultants.
Figures
References
-
- Garden E, Tomer N, Al-Alao O, et al. . MP28-09 contemporary trends in utilization and Medicare reimbursement for ambulatory BPH procedures (2014–2018). J Urol 2021;206(Supplement 3):e484.. https://www.auajournals.org/doi/pdf/10.1097/JU.0000000000002025.09
-
- Dalimov Z, Hamann H, Alavi-Dunn Nicole, et al. . PD29-09 trends in minimally invasive surgical therapies for benign prostatic hyperplasia: Treatment substitution or treatment expansion effect by prostatic urethral lift? J Urol 2020;203(Supplement 4S):e621.. https://www.auajournals.org/doi/pdf/10.1097/JU.0000000000000893.09
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
