The Histidine Kinase CckA Is Directly Inhibited by a Response Regulator-like Protein in a Negative Feedback Loop
- PMID: 35876508
- PMCID: PMC9430884
- DOI: 10.1128/mbio.01481-22
The Histidine Kinase CckA Is Directly Inhibited by a Response Regulator-like Protein in a Negative Feedback Loop
Abstract
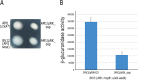
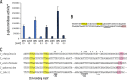
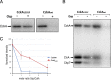
In alphaproteobacteria, the two-component system (TCS) formed by the hybrid histidine kinase CckA, the phosphotransfer protein ChpT, and the response regulator CtrA is widely distributed. In these microorganisms, this system controls diverse functions such as motility, DNA repair, and cell division. In Caulobacterales and Rhizobiales, CckA is regulated by the pseudo- histidine kinase DivL, and the response regulator DivK. However, this regulatory circuit differs for other bacterial groups. For instance, in Rhodobacterales, DivK is absent and DivL consists of only the regulatory PAS domain. In this study, we report that, in Rhodobacter sphaeroides, the kinase activity of CckA is inhibited by Osp, a single domain response regulator (SDRR) protein that directly interacts with the transmitter domain of CckA. In vitro, the kinase activity of CckA was severely inhibited with an equimolar amount of Osp, whereas the phosphatase activity of CckA was not affected. We also found that the expression of osp is activated by CtrA creating a negative feedback loop. However, under growth conditions known to activate the TCS, the increased expression of osp does not parallel Osp accumulation, indicating a complex regulation. Phylogenetic analysis of selected species of Rhodobacterales revealed that Osp is widely distributed in several genera. For most of these species, we found a sequence highly similar to the CtrA-binding site in the control region of osp, suggesting that the TCS CckA/ChpT/CtrA is controlled by a novel regulatory circuit that includes Osp in these bacteria. IMPORTANCE The two-component systems (TCS) in bacteria in its simplest architecture consist of a histidine kinase (HK) and a response regulator (RR). In response to a specific stimulus, the HK is activated and drives phosphorylation of the RR, which is responsible of generating an adaptive response. These systems are ubiquitous among bacteria and are frequently controlled by accessory proteins. In alphaproteobacteria, the TCS formed by the HK CckA, the phosphotransferase ChpT, and the RR CtrA is widely distributed. Currently, most of the information of this system and its regulatory proteins comes from findings carried out in microorganisms where it is essential. However, this is not the case in many species, and studies of this TCS and its regulatory proteins are lacking. In this study, we found that Osp, a RR-like protein, inhibits the kinase activity of CckA in a negative feedback loop since osp expression is activated by CtrA. The inhibitory role of Osp and the similar action of the previously reported FixT protein, suggests the existence of a new group of RR-like proteins whose main function is to interact with the HK and prevent its phosphorylation.
Keywords: CckA; Osp; Rhodobacter sphaeroides; Roseobacteraceae; bacterial signal transduction; hybrid histidine kinase; two-component systems.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

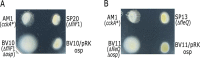







Similar articles
-
Repression of ctrA and chpT by a transcriptional regulator of the Xre family that is expressed by RpoN3 and its cognate activator protein in Cereibacter sphaeroides.PLoS One. 2025 Apr 15;20(4):e0321186. doi: 10.1371/journal.pone.0321186. eCollection 2025. PLoS One. 2025. PMID: 40233053 Free PMC article.
-
The CckA-ChpT-CtrA Phosphorelay Controlling Rhodobacter capsulatus Gene Transfer Agent Production Is Bidirectional and Regulated by Cyclic di-GMP.J Bacteriol. 2021 Feb 8;203(5):e00525-20. doi: 10.1128/JB.00525-20. Print 2021 Feb 8. J Bacteriol. 2021. PMID: 33288624 Free PMC article.
-
The Protease ClpXP and the PAS Domain Protein DivL Regulate CtrA and Gene Transfer Agent Production in Rhodobacter capsulatus.Appl Environ Microbiol. 2018 May 17;84(11):e00275-18. doi: 10.1128/AEM.00275-18. Print 2018 Jun 1. Appl Environ Microbiol. 2018. PMID: 29625982 Free PMC article.
-
Recent advances in the Phos-tag technique focused on the analysis of phosphoproteins in a bacterial two-component system.J Proteomics. 2022 Feb 10;252:104429. doi: 10.1016/j.jprot.2021.104429. Epub 2021 Nov 20. J Proteomics. 2022. PMID: 34813946 Review.
-
Regulation of gene expression by non-phosphorylated response regulators.Int Microbiol. 2021 Nov;24(4):521-529. doi: 10.1007/s10123-021-00180-2. Epub 2021 May 13. Int Microbiol. 2021. PMID: 33987704 Review.
Cited by
-
Rotation of the Fla2 flagella of Cereibacter sphaeroides requires the periplasmic proteins MotK and MotE that interact with the flagellar stator protein MotB2.PLoS One. 2024 Mar 20;19(3):e0298028. doi: 10.1371/journal.pone.0298028. eCollection 2024. PLoS One. 2024. PMID: 38507361 Free PMC article.
-
CerM and Its Antagonist CerN Are New Components of the Quorum Sensing System in Cereibacter sphaeroides, Signaling to the CckA/ChpT/CtrA System.Microbiologyopen. 2024 Dec;13(6):e012. doi: 10.1002/mbo3.70012. Microbiologyopen. 2024. PMID: 39696824 Free PMC article.
-
Repression of ctrA and chpT by a transcriptional regulator of the Xre family that is expressed by RpoN3 and its cognate activator protein in Cereibacter sphaeroides.PLoS One. 2025 Apr 15;20(4):e0321186. doi: 10.1371/journal.pone.0321186. eCollection 2025. PLoS One. 2025. PMID: 40233053 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
