Diet in a global cohort of adults with HIV at low-to-moderate traditional cardiovascular disease risk
- PMID: 35876637
- PMCID: PMC9612704
- DOI: 10.1097/QAD.0000000000003344
Diet in a global cohort of adults with HIV at low-to-moderate traditional cardiovascular disease risk
Abstract
Objective: To characterize diet quality across a global cohort of people with HIV (PWH).
Design: Cross-sectional analysis.
Methods: Leveraging REPRIEVE data from baseline across five Global Burden of Disease (GBD) regions, we analyzed participant responses to the Rapid Eating Assessment for Participants questionnaire. An overall diet quality score and scores for specific diet components were generated. Higher scores indicate better diet quality.
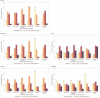
Results: Among 7736 participants (median age 50 years, 30% women, median BMI 25.8 kg/m 2 ) overall diet quality score (max score 30) was optimal in 13% of participants and good, suboptimal or poor in 45%, 38%, and 4% of participants, respectively; saturated fat score (max score 18) was good, suboptimal, or poor in 38%, 40%, or 7% of participants, respectively. Diet quality scores differed across GBD region with the highest scores reported in the South Asia region [median 23 (21-25)] and lowest in the sub-Saharan Africa region [median 15 (12-18)]; 61% of participants in the South Asia region reported optimal diet quality compared with only 6% in the sub-Saharan Africa region. Higher atherosclerotic cardiovascular risk scores were seen with worsening diet quality.
Conclusion: Among PWH eligible for primary CVD prevention, diet quality was suboptimal or poor for almost half of participants, and there were substantial variations in diet quality reported by GBD region.
Trial registration: NCT02344290.
Copyright © 2022 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
K.V.F., S.A.M., E.S.F., E.M.K., L.E.M., G.S.B., A.A.D., G.P., N.K., K.R., R.M., P.S.D., and H.J.R. have no disclosures to report; K.M.E. reports grants and personal fees from Gilead, personal fees from ViiV, Janssen, and Theratechnologies, outside the submitted work (all paid to her institution); E.T.O. reports personal fees and nonfinancial support from ViiV, personal fees and nonfinancial support from Merck, nonfinancial support from Bavarian Nordic, personal fees and nonfinancial support from Gilead, nonfinancial support from Janssen, outside the submitted work; M.V.Z. reports serving as PI of an investigator-initiated research grant from Gilead to Institution (MGH); C.F. reports grants from Gilead Sciences, ViiV Healthcare, GSK, Merck, Janssen, and Amgen unrelated to this work; J.A.A. reports grants from Atea, Emergent Biosolutions, Frontier Technologies, Gilead Sciences, grants and personal fees from Glaxo Smith Kline, grants from Janssen, grants and personal fees from Merck, grants from Pfizer, grants from Regeneron, grants from ViiV Healthcare, outside the submitted work; R.M.N. reports personal fees from Gilead Sciences, personal fees from ViiV Pharma, outside the submitted work; S.P.F. reports personal fees from Gilead Sciences unrelated to this work; S.K.G. reports grants and personal fees from Theratechnologies, grants and personal fees from ViiV, grants from Gilead, grants from Kowa, during the conduct of the study.