High Circulating Levels of the Homeostatic Chemokines CCL19 and CCL21 Predict Mortality and Disease Severity in COVID-19
- PMID: 35876699
- PMCID: PMC9384496
- DOI: 10.1093/infdis/jiac313
High Circulating Levels of the Homeostatic Chemokines CCL19 and CCL21 Predict Mortality and Disease Severity in COVID-19
Abstract
Background: Immune dysregulation is a major factor in the development of severe coronavirus disease 2019 (COVID-19). The homeostatic chemokines CCL19 and CCL21 have been implicated as mediators of tissue inflammation, but data on their regulation in severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection is limited. We thus investigated the levels of these chemokines in COVID-19 patients.
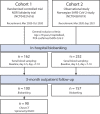
Methods: Serial blood samples were obtained from patients hospitalized with COVID-19 (n = 414). Circulating CCL19 and CCL21 levels during hospitalization and 3-month follow-up were analyzed. In vitro assays and analysis of RNAseq data from public repositories were performed to further explore possible regulatory mechanisms.
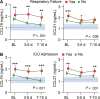
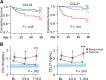
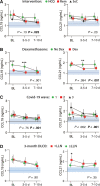
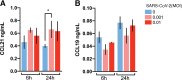
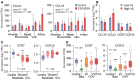
Results: A consistent increase in circulating levels of CCL19 and CCL21 was observed, with high levels correlating with disease severity measures, including respiratory failure, need for intensive care, and 60-day all-cause mortality. High levels of CCL21 at admission were associated with persisting impairment of pulmonary function at the 3-month follow-up.
Conclusions: Our findings highlight CCL19 and CCL21 as markers of immune dysregulation in COVID-19. This may reflect aberrant regulation triggered by tissue inflammation, as observed in other chronic inflammatory and autoimmune conditions. Determination of the source and regulation of these chemokines and their effects on lung tissue is warranted to further clarify their role in COVID-19.
Clinical trials registration: NCT04321616 and NCT04381819.
Keywords: SARS-CoV-2; chemokine; predictive markers; respiratory distress syndrome; viral infection.
© The Author(s) 2022. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

