Deep Learning vs Traditional Breast Cancer Risk Models to Support Risk-Based Mammography Screening
- PMID: 35876790
- PMCID: PMC9552206
- DOI: 10.1093/jnci/djac142
Deep Learning vs Traditional Breast Cancer Risk Models to Support Risk-Based Mammography Screening
Abstract
Background: Deep learning breast cancer risk models demonstrate improved accuracy compared with traditional risk models but have not been prospectively tested. We compared the accuracy of a deep learning risk score derived from the patient's prior mammogram to traditional risk scores to prospectively identify patients with cancer in a cohort due for screening.
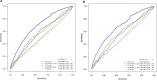
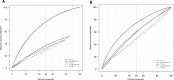
Methods: We collected data on 119 139 bilateral screening mammograms in 57 617 consecutive patients screened at 5 facilities between September 18, 2017, and February 1, 2021. Patient demographics were retrieved from electronic medical records, cancer outcomes determined through regional tumor registry linkage, and comparisons made across risk models using Wilcoxon and Pearson χ2 2-sided tests. Deep learning, Tyrer-Cuzick, and National Cancer Institute Breast Cancer Risk Assessment Tool (NCI BCRAT) risk models were compared with respect to performance metrics and area under the receiver operating characteristic curves.
Results: Cancers detected per thousand patients screened were higher in patients at increased risk by the deep learning model (8.6, 95% confidence interval [CI] = 7.9 to 9.4) compared with Tyrer-Cuzick (4.4, 95% CI = 3.9 to 4.9) and NCI BCRAT (3.8, 95% CI = 3.3 to 4.3) models (P < .001). Area under the receiver operating characteristic curves of the deep learning model (0.68, 95% CI = 0.66 to 0.70) was higher compared with Tyrer-Cuzick (0.57, 95% CI = 0.54 to 0.60) and NCI BCRAT (0.57, 95% CI = 0.54 to 0.60) models. Simulated screening of the top 50th percentile risk by the deep learning model captured statistically significantly more patients with cancer compared with Tyrer-Cuzick and NCI BCRAT models (P < .001).
Conclusions: A deep learning model to assess breast cancer risk can support feasible and effective risk-based screening and is superior to traditional models to identify patients destined to develop cancer in large screening cohorts.
© The Author(s) 2022. Published by Oxford University Press. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures
Comment in
-
Cancer Risk Prediction Paradigm Shift: Using Artificial Intelligence to Improve Performance and Health Equity.J Natl Cancer Inst. 2022 Oct 6;114(10):1317-1319. doi: 10.1093/jnci/djac143. J Natl Cancer Inst. 2022. PMID: 35876797 Free PMC article. No abstract available.
-
Beyond the AJR: Deep Learning Model for Risk-Based Breast Cancer Screening.AJR Am J Roentgenol. 2023 Jul;221(1):141. doi: 10.2214/AJR.22.28703. Epub 2022 Nov 9. AJR Am J Roentgenol. 2023. PMID: 36350116 No abstract available.
References
-
- U.S. Health and Human Services website. Health and Human Services: re-opening approach. https://www.mass.gov/doc/eohhs-health-care-phase-1-reopening-approach-ma.... Published May 2020. Accessed March 2022.
-
- Society of Breast Imaging website. SBI recommendations for a thoughtful return to caring for patients. https://www.scirp.org/reference/referencespapers.aspx?referenceid=2774635. Published July 9, 2020. Accessed July 28, 2022.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

