Electron tomography of prolamellar bodies and their transformation into grana thylakoids in cryofixed Arabidopsis cotyledons
- PMID: 35876816
- PMCID: PMC9516191
- DOI: 10.1093/plcell/koac205
Electron tomography of prolamellar bodies and their transformation into grana thylakoids in cryofixed Arabidopsis cotyledons
Abstract
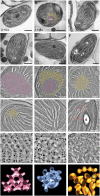
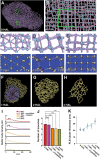
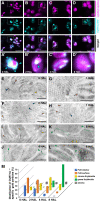
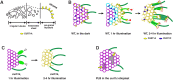
The para-crystalline structures of prolamellar bodies (PLBs) and light-induced etioplast-to-chloroplast transformation have been investigated via electron microscopy. However, such studies suffer from chemical fixation artifacts and limited volumes of 3D reconstruction. Here, we examined Arabidopsis thaliana cotyledon cells by electron tomography (ET) to visualize etioplasts and their conversion into chloroplasts. We employed scanning transmission ET to image large volumes and high-pressure freezing to improve sample preservation. PLB tubules were arranged in a zinc blende-type lattice-like carbon atoms in diamonds. Within 2 h after illumination, the lattice collapsed from the PLB exterior and the disorganized tubules merged to form thylakoid sheets (pre-granal thylakoids), which folded and overlapped with each other to create grana stacks. Since the nascent pre-granal thylakoids contained curved membranes in their tips, we examined the expression and localization of CURT1 (CURVATURE THYLAKOID1) proteins. CURT1A transcripts were most abundant in de-etiolating cotyledon samples, and CURT1A was concentrated at the PLB periphery. In curt1a etioplasts, PLB-associated thylakoids were swollen and failed to form grana stacks. In contrast, PLBs had cracks in their lattices in curt1c etioplasts. Our data provide evidence that CURT1A is required for pre-granal thylakoid assembly from PLB tubules during de-etiolation, while CURT1C contributes to cubic crystal growth in the dark.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
Curvature thylakoid 1 proteins modulate prolamellar body morphology and promote organized thylakoid biogenesis in Arabidopsis thaliana.Proc Natl Acad Sci U S A. 2021 Oct 19;118(42):e2113934118. doi: 10.1073/pnas.2113934118. Proc Natl Acad Sci U S A. 2021. PMID: 34654749 Free PMC article.
-
Thylakoid-Bound Polysomes and a Dynamin-Related Protein, FZL, Mediate Critical Stages of the Linear Chloroplast Biogenesis Program in Greening Arabidopsis Cotyledons.Plant Cell. 2018 Jul;30(7):1476-1495. doi: 10.1105/tpc.17.00972. Epub 2018 Jun 7. Plant Cell. 2018. PMID: 29880711 Free PMC article.
-
Arabidopsis CURVATURE THYLAKOID1 proteins modify thylakoid architecture by inducing membrane curvature.Plant Cell. 2013 Jul;25(7):2661-78. doi: 10.1105/tpc.113.113118. Epub 2013 Jul 9. Plant Cell. 2013. PMID: 23839788 Free PMC article.
-
A brief history of how microscopic studies led to the elucidation of the 3D architecture and macromolecular organization of higher plant thylakoids.Photosynth Res. 2020 Sep;145(3):237-258. doi: 10.1007/s11120-020-00782-3. Epub 2020 Oct 5. Photosynth Res. 2020. PMID: 33017036 Free PMC article. Review.
-
Dynamic flexibility in the structure and function of photosystem II in higher plant thylakoid membranes: the grana enigma.Photosynth Res. 2008 Oct-Dec;98(1-3):575-87. doi: 10.1007/s11120-008-9381-3. Epub 2008 Nov 8. Photosynth Res. 2008. PMID: 18998237 Review.
Cited by
-
Observation of Vacuoles in Arabidopsis Developing Pollen Grains with Whole-Cell Electron Tomography.Methods Mol Biol. 2024;2841:171-178. doi: 10.1007/978-1-0716-4059-3_16. Methods Mol Biol. 2024. PMID: 39115776
-
Lipid polymorphism of plant thylakoid membranes. The dynamic exchange model - facts and hypotheses.Physiol Plant. 2025 Mar-Apr;177(2):e70230. doi: 10.1111/ppl.70230. Physiol Plant. 2025. PMID: 40251902 Free PMC article. Review.
-
Vacuolar degradation of plant organelles.Plant Cell. 2024 Sep 3;36(9):3036-3056. doi: 10.1093/plcell/koae128. Plant Cell. 2024. PMID: 38657116 Free PMC article. Review.
-
Plantorganelle Hunter is an effective deep-learning-based method for plant organelle phenotyping in electron microscopy.Nat Plants. 2023 Oct;9(10):1760-1775. doi: 10.1038/s41477-023-01527-5. Epub 2023 Sep 25. Nat Plants. 2023. PMID: 37749240
-
De-etiolation is Almost Color Blind: The Study of Photosynthesis Awakening under Blue and Red Light.Plant Cell Physiol. 2024 Dec 21;65(12):1993-2017. doi: 10.1093/pcp/pcae119. Plant Cell Physiol. 2024. PMID: 39392728 Free PMC article.
References
-
- Blomqvist LA, Ryberg M, Sundqvist C (2008) Proteomic analysis of highly purified prolamellar bodies reveals their significance in chloroplast development. Photosynthesis Res 96: 37–50 - PubMed
-
- Cotton FA, Wilkinson G, Gaus PL (1995) Basic inorganic chemistry. InIonic solids, John Wiley & Sons, New York, p 139
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

