ADP-ribosylation factor D1 modulates Golgi morphology, cell plate formation, and plant growth in Arabidopsis
- PMID: 35876822
- PMCID: PMC9516763
- DOI: 10.1093/plphys/kiac329
ADP-ribosylation factor D1 modulates Golgi morphology, cell plate formation, and plant growth in Arabidopsis
Abstract
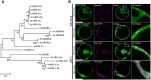
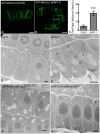
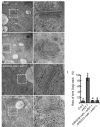
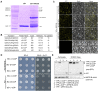
ADP-ribosylation factor (ARF) family proteins, one type of small guanine-nucleotide-binding (G) proteins, play a central role in regulating vesicular traffic and organelle structures in eukaryotes. The Arabidopsis (Arabidopsis thaliana) genome contains more than 21 ARF proteins, but relatively little is known about the functional heterogeneity of ARF homologs in plants. Here, we characterized the function of a unique ARF protein, ARFD1B, in Arabidopsis. ARFD1B exhibited both cytosol and punctate localization patterns, colocalizing with a Golgi marker in protoplasts and transgenic plants. Distinct from other ARF1 homologs, overexpression of a dominant-negative mutant form of ARFD1B did not alter the localization of the Golgi marker mannosidase I (ManI)-RFP in Arabidopsis cells. Interestingly, the ARFD1 artificial microRNA knockdown mutant arfd1 displayed a deleterious growth phenotype, while this phenotype was restored in complemented plants. Further, confocal imaging and transmission electron microscopy analyses of the arfd1 mutant revealed defective cell plate formation and abnormal Golgi morphology. Pull-down and liquid chromatography-tandem mass spectrometry analyses identified Coat Protein I (COPI) components as interacting partners of ARFD1B, and subsequent bimolecular fluorescence complementation, yeast (Saccharomyces cerevisiae) two-hybrid, and co-immunoprecipitation assays further confirmed these interactions. These results demonstrate that ARFD1 is required for cell plate formation, maintenance of Golgi morphology, and plant growth in Arabidopsis.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures



Similar articles
-
The role of ADP-ribosylation factor and SAR1 in vesicular trafficking in plants.Biochim Biophys Acta. 2004 Jul 1;1664(1):9-30. doi: 10.1016/j.bbamem.2004.04.005. Biochim Biophys Acta. 2004. PMID: 15238254 Review.
-
Recruitment of Arf1-GDP to Golgi by Glo3p-type ArfGAPs is crucial for golgi maintenance and plant growth.Plant Physiol. 2013 Feb;161(2):676-91. doi: 10.1104/pp.112.209148. Epub 2012 Dec 24. Plant Physiol. 2013. PMID: 23266962 Free PMC article.
-
A COPI coat subunit interacts directly with an early-Golgi localized Arf exchange factor.EMBO Rep. 2009 Jan;10(1):58-64. doi: 10.1038/embor.2008.221. Epub 2008 Nov 28. EMBO Rep. 2009. PMID: 19039328 Free PMC article.
-
ADP-ribosylation factor 1 of Arabidopsis plays a critical role in intracellular trafficking and maintenance of endoplasmic reticulum morphology in Arabidopsis.Plant Physiol. 2002 Aug;129(4):1507-20. doi: 10.1104/pp.003624. Plant Physiol. 2002. PMID: 12177464 Free PMC article.
-
ARF1 regulatory factors and COPI vesicle formation.Curr Opin Cell Biol. 2002 Aug;14(4):423-7. doi: 10.1016/s0955-0674(02)00346-0. Curr Opin Cell Biol. 2002. PMID: 12383792 Review.
Cited by
-
Characterization of the small Arabidopsis thaliana GTPase and ADP-ribosylation factor-like 2 protein TITAN 5.J Cell Sci. 2024 Aug 1;137(15):jcs262315. doi: 10.1242/jcs.262315. Epub 2024 Aug 14. J Cell Sci. 2024. PMID: 39056156 Free PMC article.
-
BrrA02.LMI1 Encodes a Homeobox Protein That Affects Leaf Margin Development in Brassica rapa.Int J Mol Sci. 2023 Sep 18;24(18):14205. doi: 10.3390/ijms241814205. Int J Mol Sci. 2023. PMID: 37762508 Free PMC article.
-
An Overview of Protein Secretion in Plant Cells.Methods Mol Biol. 2024;2841:19-36. doi: 10.1007/978-1-0716-4059-3_2. Methods Mol Biol. 2024. PMID: 39115762 Review.
-
Fusion gene 4CL-CCR promotes lignification in tobacco suspension cells.Plant Cell Rep. 2023 May;42(5):939-952. doi: 10.1007/s00299-023-03002-z. Epub 2023 Mar 25. Plant Cell Rep. 2023. PMID: 36964306
-
Characterization and expression analysis of the B3 gene family during seed development in Akebia trifoliata.BMC Genomics. 2024 Nov 9;25(1):1060. doi: 10.1186/s12864-024-10981-0. BMC Genomics. 2024. PMID: 39516780 Free PMC article.
References
-
- Aoyama T, Chua NH (1997) A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J 11: 605–612 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

