Pyruvate, phosphate dikinase regulatory protein impacts light response of C4 photosynthesis in Setaria viridis
- PMID: 35876823
- PMCID: PMC9516741
- DOI: 10.1093/plphys/kiac333
Pyruvate, phosphate dikinase regulatory protein impacts light response of C4 photosynthesis in Setaria viridis
Abstract
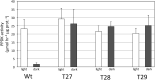
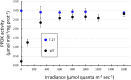
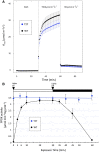
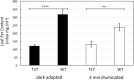
In C4 plants, the pyruvate (Pyr), phosphate dikinase regulatory protein (PDRP) regulates the activity of the C4 pathway enzyme Pyr, phosphate dikinase (PPDK) in a light-/dark-dependent manner. The importance of this regulatory action to C4 pathway function and overall C4 photosynthesis is unknown. To resolve this question, we assessed in vivo PPDK phospho-regulation and whole leaf photophysiology in a CRISPR-Cas9 PDRP knockout (KO) mutant of the NADP-ME C4 grass green millet (Setaria viridis). PDRP enzyme activity was undetectable in leaf extracts from PDRP KO lines. Likewise, PPDK phosphorylated at the PDRP-regulatory Thr residue was immunologically undetectable in leaf extracts. PPDK enzyme activity in rapid leaf extracts was constitutively high in the PDRP KO lines, irrespective of light or dark pretreatment of leaves. Gas exchange analysis of net CO2 assimilation revealed PDRP KO leaves had markedly slower light induction kinetics when leaves transition from dark to high-light or low-light to high-light. In the initial 30 min of the light induction phase, KO leaves had an ∼15% lower net CO2 assimilation rate versus the wild-type (WT). Despite the impaired slower induction kinetics, we found growth and vigor of the KO lines to be visibly indistinguishable from the WT when grown in normal air and under standard growth chamber conditions. However, the PDRP KO plants grown under a fluctuating light regime exhibited a gradual multi-day decline in Fv/Fm, indicative of progressive photosystem II damage due to the absence of PDRP. Collectively, our results demonstrate that one of PDRP's functions in C4 photosynthesis is to ensure optimal photosynthetic light induction kinetics during dynamic changes in incident light.
© American Society of Plant Biologists 2022. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Figures





Similar articles
-
Cool C4 photosynthesis: pyruvate Pi dikinase expression and activity corresponds to the exceptional cold tolerance of carbon assimilation in Miscanthus x giganteus.Plant Physiol. 2008 Sep;148(1):557-67. doi: 10.1104/pp.108.120709. Epub 2008 Jun 6. Plant Physiol. 2008. PMID: 18539777 Free PMC article.
-
Morphophysiological alterations in transgenic rice lines expressing PPDK and ME genes from the C4 model Setaria italica.J Plant Physiol. 2021 Sep;264:153482. doi: 10.1016/j.jplph.2021.153482. Epub 2021 Jul 21. J Plant Physiol. 2021. PMID: 34330009
-
Structural Basis of Reversible Phosphorylation by Maize Pyruvate Orthophosphate Dikinase Regulatory Protein.Plant Physiol. 2016 Feb;170(2):732-41. doi: 10.1104/pp.15.01709. Epub 2015 Nov 30. Plant Physiol. 2016. PMID: 26620526 Free PMC article.
-
The gene for pyruvate, orthophosphate dikinase in C4 plants: structure, regulation and evolution.Plant Cell Physiol. 1995 Sep;36(6):937-43. doi: 10.1093/oxfordjournals.pcp.a078864. Plant Cell Physiol. 1995. PMID: 8528609 Review.
-
What can enzymes of C₄ photosynthesis do for C₃ plants under stress?Plant Sci. 2011 Apr;180(4):575-83. doi: 10.1016/j.plantsci.2010.12.005. Epub 2010 Dec 15. Plant Sci. 2011. PMID: 21421406 Review.
Cited by
-
Autophagy: a game changer for plant development and crop improvement.Planta. 2022 Oct 28;256(6):103. doi: 10.1007/s00425-022-04004-z. Planta. 2022. PMID: 36307739 Review.
-
Prospects and perspectives: inferring physiological and regulatory targets for CAM from molecular and modelling approaches.Ann Bot. 2023 Nov 25;132(4):583-596. doi: 10.1093/aob/mcad142. Ann Bot. 2023. PMID: 37742290 Free PMC article. Review.
-
Effects of Environmental and Non-Environmental Factors on Dynamic Photosynthetic Carbon Assimilation in Leaves under Changing Light.Plants (Basel). 2023 May 18;12(10):2015. doi: 10.3390/plants12102015. Plants (Basel). 2023. PMID: 37653932 Free PMC article. Review.
-
Molecular mechanisms and genetic regulation of self-incompatibility in flowering plants: implications for crop improvement and evolutionary biology.Plant Mol Biol. 2025 Jun 25;115(4):76. doi: 10.1007/s11103-025-01610-9. Plant Mol Biol. 2025. PMID: 40560319 Review.
-
Photosynthesis, Water Status and K+/Na+ Homeostasis of Buchoe dactyloides Responding to Salinity.Plants (Basel). 2023 Jun 27;12(13):2459. doi: 10.3390/plants12132459. Plants (Basel). 2023. PMID: 37447020 Free PMC article.
References
-
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD (1990) Enzymes of C4 photosynthesis. InLea PJ, ed, Methods in Plant Biochemistry, Vol 3, Academic Press, San Diego, CA, pp 39–72
-
- Burnell JN, Hatch MD (1985) Regulation of C4 photosynthesis: purification and properties of the protein catalyzing ADP-mediated inactivation and Pi-mediated activation of pyruvate, Pi dikinase. Arch Biochem Biophys 237: 490–503 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

