How reduced are nucleophilic gold complexes?
- PMID: 35877065
- PMCID: PMC9764324
- DOI: 10.1039/d2dt01694j
How reduced are nucleophilic gold complexes?
Abstract
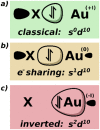
Nucleophilic formal gold(-I) and gold(I) complexes are investigated via Intrinsic Bond Orbital analysis and Energy Decomposition Analysis, based on density functional theory calculations. The results indicate gold(0) centres engaging in electron-sharing bonding with Al- and B- based ligands. Multiconfigurational (CASSCF) calculations corroborate the findings, highlighting the gap between the electonic structures and the oxidation state formalism.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

