Docetaxel for Nonmetastatic Prostate Cancer: Long-Term Survival Outcomes in the STAMPEDE Randomized Controlled Trial
- PMID: 35877084
- PMCID: PMC9338456
- DOI: 10.1093/jncics/pkac043
Docetaxel for Nonmetastatic Prostate Cancer: Long-Term Survival Outcomes in the STAMPEDE Randomized Controlled Trial
Abstract
Background: STAMPEDE previously reported adding upfront docetaxel improved overall survival for prostate cancer patients starting long-term androgen deprivation therapy. We report long-term results for non-metastatic patients using, as primary outcome, metastatic progression-free survival (mPFS), an externally demonstrated surrogate for overall survival.
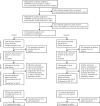
Methods: Standard of care (SOC) was androgen deprivation therapy with or without radical prostate radiotherapy. A total of 460 SOC and 230 SOC plus docetaxel were randomly assigned 2:1. Standard survival methods and intention to treat were used. Treatment effect estimates were summarized from adjusted Cox regression models, switching to restricted mean survival time if non-proportional hazards. mPFS (new metastases, skeletal-related events, or prostate cancer death) had 70% power (α = 0.05) for a hazard ratio (HR) of 0.70. Secondary outcome measures included overall survival, failure-free survival (FFS), and progression-free survival (PFS: mPFS, locoregional progression).
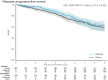
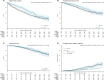
Results: Median follow-up was 6.5 years with 142 mPFS events on SOC (3 year and 54% increases over previous report). There was no good evidence of an advantage to SOC plus docetaxel on mPFS (HR = 0.89, 95% confidence interval [CI] = 0.66 to 1.19; P = .43); with 5-year mPFS 82% (95% CI = 78% to 87%) SOC plus docetaxel vs 77% (95% CI = 73% to 81%) SOC. Secondary outcomes showed evidence SOC plus docetaxel improved FFS (HR = 0.70, 95% CI = 0.55 to 0.88; P = .002) and PFS (nonproportional P = .03, restricted mean survival time difference = 5.8 months, 95% CI = 0.5 to 11.2; P = .03) but no good evidence of overall survival benefit (125 SOC deaths; HR = 0.88, 95% CI = 0.64 to 1.21; P = .44). There was no evidence SOC plus docetaxel increased late toxicity: post 1 year, 29% SOC and 30% SOC plus docetaxel grade 3-5 toxicity.
Conclusions: There is robust evidence that SOC plus docetaxel improved FFS and PFS (previously shown to increase quality-adjusted life-years), without excess late toxicity, which did not translate into benefit for longer-term outcomes. This may influence patient management in individual cases.
© The Author(s) 2022. Published by Oxford University Press.
Figures

Comment in
-
Intensification of Androgen Deprivation Therapy in High-Risk, Nonmetastatic Prostate Cancer: Lessons From STAMPEDE.JNCI Cancer Spectr. 2022 Jul 1;6(4):pkac044. doi: 10.1093/jncics/pkac044. JNCI Cancer Spectr. 2022. PMID: 35877083 Free PMC article. No abstract available.
References
-
- James ND, Sydes MR, Clarke NW, et al.Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177. doi:10.1016/S0140-6736(15)01037-5. - DOI - PMC - PubMed
-
- Fizazi K, Faivre L, Lesaunier F, et al.Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16(7):787-794. doi:10.1016/S1470-2045(15)00011-X. - DOI - PubMed
-
- Gravis G, Boher JM, Joly F, et al.GETUG. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur Urol. 2016;70(2):256-262. doi:10.1016/j.eururo.2015.11.005. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
